|
Argon Dimer Potential
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all of the argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a mixture o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incandescent Light Bulb
An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated until it glows. The filament is enclosed in a glass bulb with a vacuum or inert gas to protect the filament from oxidation. Current is supplied to the filament by terminals or wires embedded in the glass. A bulb socket provides mechanical support and electrical connections. Incandescent bulbs are manufactured in a wide range of sizes, light output, and voltage ratings, from 1.5 volts to about 300 volts. They require no external regulating equipment, have low manufacturing costs, and work equally well on either alternating current or direct current. As a result, the incandescent bulb became widely used in household and commercial lighting, for portable lighting such as table lamps, car headlamps, and flashlights, and for decorative and advertising lighting. Incandescent bulbs are much less efficient than other types of electric lighting, converting les ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
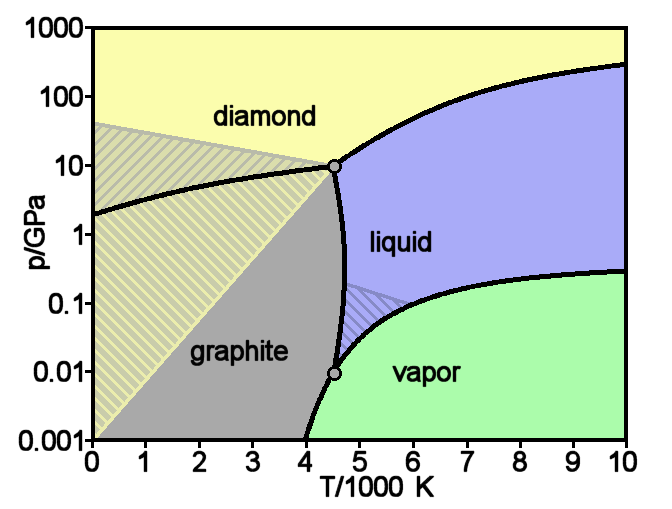
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shielding Gas
Shielding gases are inert or semi-inert gases that are commonly used in several welding processes, most notably gas metal arc welding and gas tungsten arc welding (GMAW and GTAW, more popularly known as MIG (Metal Inert Gas) and TIG (Tungsten Inert Gas), respectively). Their purpose is to protect the weld area from oxygen, and water vapour. Depending on the materials being welded, these atmospheric gases can reduce the quality of the weld or make the welding more difficult. Other arc welding processes use alternative methods of protecting the weld from the atmosphere as well – shielded metal arc welding, for example, uses an electrode covered in a flux (metallurgy), flux that produces carbon dioxide when consumed, a semi-inert gas that is an acceptable shielding gas for welding steel. Improper choice of a welding gas can lead to a porous and weak weld, or to excessive spatter; the latter, while not affecting the weld itself, causes loss of productivity due to the labor needed to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inert Gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to as the inert gases. Inert gases are used generally to avoid unwanted chemical reactions degrading a sample. These undesirable chemical reactions are often oxidation and hydrolysis reactions with the oxygen and moisture in air. The term ''inert gas'' is context-dependent because several of the noble gases can be made to react under certain conditions. Purified argon gas is the most commonly used inert gas due to its high natural abundance (78.3% N2, 1% Ar in air) and low relative cost. Unlike noble gases, an inert gas is not necessarily elemental and is often a compound gas. Like the noble gases, the tendency for non-reactivity is due to the valence, the outermost electron shell, being complete in all the inert gases. This is a tendency, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Air
Liquid air is air that has been cooled to very low temperatures ( cryogenic temperatures), so that it has condensed into a pale blue mobile liquid. To thermally insulate it from room temperature, it is stored in specialized containers ( vacuum insulated flasks are often used). Liquid air can absorb heat rapidly and revert to its gaseous state. It is often used for condensing other substances into liquid and/or solidifying them, and as an industrial source of nitrogen, oxygen, argon, and other inert gases through a process called air separation. Properties Liquid air has a density of approximately . The density of a given air sample varies depending on the composition of that sample (e.g. humidity & concentration). Since dry gaseous air contains approximately 78% nitrogen, 21% oxygen, and 1% argon, the density of liquid air at standard composition is calculated by the percentage of the components and their respective liquid densities (see liquid nitrogen and liquid oxygen). Alth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil. Laboratory setup Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a Bunsen burner, a round-bottomed flask and a condenser, as well as the single-purpose fractionating column. As an example, consider the distillation of a mixture of water and ethanol. Ethanol boils at while water boils at . So, by heating the mixture, the most volatile component (ethanol) will concen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Temperature Scale Of 1990
The International Temperature Scale of 1990 (ITS-90) is an equipment calibration standard specified by the International Committee of Weights and Measures (CIPM) for making measurements on the Kelvin and Celsius temperature scales. It is an approximation of thermodynamic temperature that facilitates the comparability and compatibility of temperature measurements internationally. It defines fourteen calibration points ranging from to ( to ) and is subdivided into multiple temperature ranges which overlap in some instances. ITS-90 is the most recent of a series of International Temperature Scales adopted by the CIPM since 1927. Adopted at the 1989 General Conference on Weights and Measures, it supersedes the International Practical Temperature Scale of 1968 (amended edition of 1975) and the 1976 "Provisional 0.5 K to 30 K Temperature Scale". The CCT has also published several online guidebooks to aid realisations of the ITS-90. The lowest temperature covered by the ITS-90 is 0.6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and physicist William Thomson, 1st Baron Kelvin (1824–1907). The Kelvin scale is an absolute thermodynamic temperature scale, meaning it uses absolute zero as its null (zero) point. Historically, the Kelvin scale was developed by shifting the starting point of the much-older Celsius scale down from the melting point of water to absolute zero, and its increments still closely approximate the historic definition of a degree Celsius, but since 2019 the scale has been defined by fixing the Boltzmann constant to be exactly . Hence, one kelvin is equal to a change in the thermodynamic temperature that results in a change of thermal energy by . The temperature in degree Celsius is now defined as the temperature in kelvins minus 273.15, meaning t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporisation curve meet. For example, the triple point of mercury occurs at a temperature of and a pressure of 0.165 m Pa. In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphs. Helium-4 is a special case that presents a triple point involving two different fluid phases (lambda point). The triple point of water was used to define the kelvin, the base unit of thermodynamic temperature in the International System of Units (SI). The value of the triple point of water was fixed by definition, rather than measured, but that changed with the 2019 redefinition of SI base units. The triple points of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octet Rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens; although more generally the rule is applicable for the s-block and p-block of the periodic table. Other rules exist for other elements, such as the duplet rule for hydrogen and helium, or the 18-electron rule for transition metals. The valence electrons can be counted using a Lewis electron dot diagram as shown at the right for carbon dioxide. The electrons shared by the two atoms in a covalent bond are counted twice, once for each atom. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon octet and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

%2C_black_and_white.png)