|
Antisense Therapy
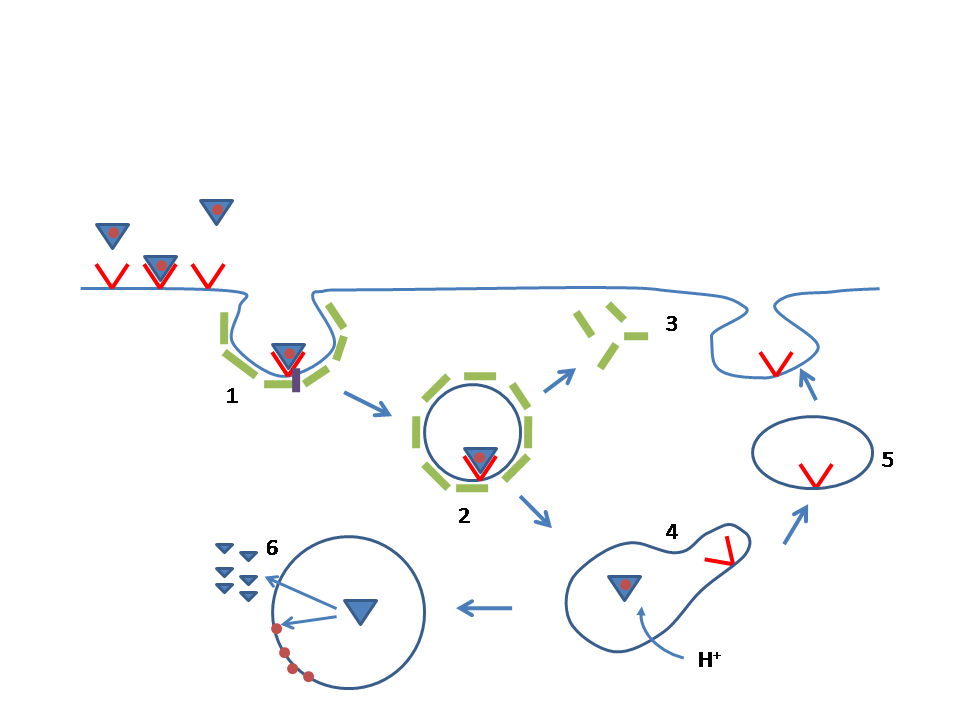
Antisense therapy is a form of treatment that uses antisense oligonucleotides (ASOs) to target messenger RNA (mRNA). ASOs are capable of altering mRNA expression through a variety of mechanisms, including ribonuclease H mediated decay of the pre-mRNA, direct steric blockage, and exon content modulation through splicing site binding on pre-mRNA. Several ASOs have been approved in the United States, the European Union, and elsewhere. Nomenclature The common stem for antisense oligonucleotides drugs is -rsen. The substem -virsen designates antiviral antisense oligonucleotides. Pharmacokinetics and pharmacodynamics Half-life and stability ASO-based drugs employ highly modified, single-stranded chains of synthetic nucleic acids that achieve wide tissue distribution with very long half-lives. For instance, many ASO-based drugs contain phosphorothioate substitutions and 2' sugar modifications to inhibit nuclease degradation enabling vehicle-free delivery to cells. ''In vivo'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligonucleotidase
Oligonucleotidase (, ''oligoribonuclease'') is an exoribonuclease derived from ''Flammulina velutipes''. This enzyme catalyses the following chemical reaction A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ... : 3'-end directed exonucleolytic cleavage of viral RNA-DNA hybrid References External links * * EC 3.1.13 {{biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eteplirsen
Eteplirsen (brand name Exondys 51) is a medication to treat, but not cure, some types of Duchenne muscular dystrophy (DMD), caused by a specific mutation. Eteplirsen only targets specific mutations and can be used to treat about 14% of DMD cases. Eteplirsen is a form of antisense therapy. Eteplirsen was developed by Steve Wilton, Sue Fletcher and colleagues at the University of Western Australia and commercialized by Sarepta Therapeutics. After a controversial debate surrounding the drug's efficacy, during which two FDA review panel members resigned in protest, eteplirsen received accelerated approval from the US Food and Drug administration in late 2016. The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) refused to authorize the use of eteplirsen. Adverse effects The following adverse events were observed in at least 10% of people who received eteplirsen in trials: vomiting, contusion, excoriation, arthralgia, rash, catheter site pain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spinal Muscular Atrophy
Spinal muscular atrophy (SMA) is a rare neuromuscular disorder that results in the loss of motor neurons and progressive muscle wasting. It is usually diagnosed in infancy or early childhood and if left untreated it is the most common genetic cause of infant death. It may also appear later in life and then have a milder course of the disease. The common feature is progressive weakness of voluntary muscles, with arm, leg and respiratory muscles being affected first. Associated problems may include poor head control, difficulties swallowing, scoliosis, and joint contractures. The age of onset and the severity of symptoms form the basis of the traditional classification of spinal muscular atrophy into a number of types. Spinal muscular atrophy is due to an abnormality (mutation) in the ''SMN1'' gene which encodes SMN, a protein necessary for survival of motor neurons. Loss of these neurons in the spinal cord prevents signalling between the brain and skeletal muscles. Another g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hereditary Transthyretin-mediated Amyloidosis
Familial amyloid polyneuropathy, also called transthyretin-related hereditary amyloidosis, transthyretin amyloidosis abbreviated also as ATTR (hereditary form), or Corino de Andrade's disease, is an autosomal dominant neurodegenerative disease. It is a form of amyloidosis, and was first identified and described by Portuguese neurologist Mário Corino da Costa Andrade, in 1952. FAP is distinct from senile systemic amyloidosis (SSA), which is not inherited, and which was determined to be the primary cause of death for 70% of supercentenarians who have been autopsied. FAP can be ameliorated by liver transplantation. Presentation Usually manifesting itself between 20 and 40 years of age, it is characterized by pain, paresthesia, muscular weakness and autonomic dysfunction. In its terminal state, the kidneys and the heart are affected. FAP is characterized by the systemic deposition of amyloidogenic variants of the transthyretin protein, especially in the peripheral nervous system, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inotersen
Inotersen, sold under the brand name Tegsedi, is a 2'-O-(2-methoxyethyl) (2'-MOE) antisense oligonucleotide medication used for the treatment of nerve damage in adults with hereditary transthyretin-mediated amyloidosis. The sequence is TCTTG GTTACATGAA ATCCC, where C is methylated C, and the first and third section (bases 1-5 and 16–20, separated from the middle section by spaces) are MOE-modified. The most common side effects are injection site reactions (redness, swelling, bleeding, pain, rash, and itching at the injection site), nausea, headache, tiredness, low platelet counts, and fever. Inotersen can cause serious side effects, including low platelet counts and kidney inflammation. Because of these serious side effects, Inotersen is available in the United States only through a restricted program called the Tegsedi Risk Evaluation and Mitigation (REMS) Program. The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication. History Inoters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The New York Times
''The New York Times'' (''the Times'', ''NYT'', or the Gray Lady) is a daily newspaper based in New York City with a worldwide readership reported in 2020 to comprise a declining 840,000 paid print subscribers, and a growing 6 million paid digital subscribers. It also is a producer of popular podcasts such as '' The Daily''. Founded in 1851 by Henry Jarvis Raymond and George Jones, it was initially published by Raymond, Jones & Company. The ''Times'' has won 132 Pulitzer Prizes, the most of any newspaper, and has long been regarded as a national " newspaper of record". For print it is ranked 18th in the world by circulation and 3rd in the U.S. The paper is owned by the New York Times Company, which is publicly traded. It has been governed by the Sulzberger family since 1896, through a dual-class share structure after its shares became publicly traded. A. G. Sulzberger, the paper's publisher and the company's chairman, is the fifth generation of the family to head the pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels of low-density lipoprotein (LDL cholesterol), in the blood and early cardiovascular disease. The most common mutations diminish the number of functional LDL receptors in the liver. Since the underlying body biochemistry is slightly different in individuals with FH, their high cholesterol levels are less responsive to the kinds of cholesterol control methods which are usually more effective in people without FH (such as dietary modification and statin tablets). Nevertheless, treatment (including higher statin doses) is usually effective. FH is classified as a type 2 familial dyslipidemia. There are five types of familial dyslipidemia (not including subtypes), and each are classified from both the altered lipid profile and by the genetic abnormality. For example, high LDL (often due to LDL receptor defect) is type 2. Others include defects in chylomicron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mipomersen
Mipomersen (INN; trade name Kynamro) is a drug used to treat homozygous familial hypercholesterolemia and is administered by subcutaneous injection. There is a serious risk of liver damage from this drug and it can only be prescribed in the context of a risk management plan. Indications Kynamro is used to treat homozygous familial hypercholesterolemia and is administered by injection. It cannot be freely prescribed; instead every person put on mipomersen is enrolled in a Risk Evaluation and Mitigation Strategies (REMS) program approved by the FDA. Pregnancy and lactation Mipomersen is pregnancy category B; women who are pregnant or intending to become pregnant should only use this drug if needed. It is unknown if it is secreted in human breast milk, but it was found to be secreted in the breast milk of rats. Contraindications The drug is contraindicated in people with moderate to severe liver impairment, active liver diseases, and unexplained high levels of transaminase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Familial Chylomicronaemia Syndrome
Familial may refer to: * ''Familial'' (album), a 2010 studio album by Phil Selway *Family, a group of people affiliated by consanguinity, affinity, or co-residence *Family (biology), one of the eight major taxonomic ranks, classified between order and genus *Heredity, passing of genetic traits to offspring **Genetic disorder, more specifically **List of genetic disorders The following is a list of genetic disorders and if known, type of mutation and for the chromosome involved. Although the parlance "disease-causing gene" is common, it is the occurrence of an abnormality in the parents that causes the impairment ... See also * * * Family (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.png)