|
Acidithiobacillus Caldus
''Acidithiobacillus caldus'' formerly belonged to the genus ''Thiobacillus'' prior to 2000, when it was reclassified along with a number of other bacterial species into one of three new genera that better categorize sulfur-oxidizing acidophiles. As a member of the Gammaproteobacteria class of Pseudomonadota, ''A. caldus'' may be identified as a Gram-negative bacterium that is frequently found in pairs. Considered to be one of the most common microbes involved in biomining, it is capable of oxidizing reduced inorganic sulfur compounds (RISCs) that form during the breakdown of sulfide minerals. The meaning of the prefix ''acidi-'' in the name ''Acidithiobacillus'' comes from the Latin word ''acidus'', signifying that members of this genus love a sour, acidic environment. ''Thio'' is derived from the Greek word ''thios'' and describes the use of sulfur as an energy source, and ''bacillus'' describes the shape of these microorganisms, which are small rods. The species name, ''caldus'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acidophiles
Acidophiles or acidophilic organisms are those that thrive under highly acidic conditions (usually at pH 5.0 or below). These organisms can be found in different branches of the tree of life, including Archaea, Bacteria,Becker, A.Types of Bacteria Living in Acidic pH" Retrieved 10 May 2017. and Eukarya. Examples A list of these organisms includes: Archaea :* Sulfolobales, an order in the Thermoproteota branch of Archaea :* Thermoplasmatales, an order in the Euryarchaeota branch of Archaea :* ARMAN, in the Euryarchaeota branch of Archaea :* ''Acidianus brierleyi, A. infernus'', facultatively anaerobic thermoacidophilic archaebacteria :* '' Halarchaeum acidiphilum'', acidophilic member of the Halobacteriacaeae :* ''Metallosphaera sedula'', thermoacidophilic Bacteria :* Acidobacteriota, a phylum of Bacteria :* Acidithiobacillales, an order of Pseudomonadota e.g. ''A. ferrooxidans, A. thiooxidans'' :*''Thiobacillus prosperus, T. acidophilus, T. organovorus, T. cuprinus'' :*'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NaCl
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 da ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Casamino Acids
Casamino acids is a mixture of amino acids and some very small peptides obtained from acid hydrolysis of casein. It is typically used in microbial growth media. It has all the essential amino acids except tryptophan, which is destroyed by digestion with sulfuric or hydrochloric acid. Casamino acids is similar to tryptone, the latter differing by being an incomplete enzymatic hydrolysis with some oligopeptides present, while casamino acids is predominantly free amino acids. Uses Casamino acids supplies a completely hydrolyzed protein nitrogen source. It contains a small amount of cystine. Tryptophan and vitamins are destroyed by the acid treatment. The remaining amino acids (in varying amounts) are a source of nutrients for various microorganisms. Amino acids are highly soluble and suitable for use in tissue culture. Salt content is typically 30-40%. Casamino acids are either found in the Daptacel brand DTaP vaccine or used in its manufacture. Appearance *White to light tan, homog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yeast Extract
Yeast extracts consist of the cell contents of yeast without the cell walls; they are used as food additives or flavorings, or as nutrients for bacterial culture media. They are often used to create savory flavors and umami taste sensations, and can be found in a large variety of packaged food, including frozen meals, crackers, snack foods, gravy, stock and more. They are rich in B vitamins (but not B12). Yeast extracts and fermented foods contain glutamic acid (free glutamates), an amino acid which adds an umami flavor. Glutamic acid is found in meat, cheese, fungi (mushrooms and yeast), and vegetables—such as broccoli, and tomatoes. The heat-autolytic process to make yeast extract of the autolysate type was invented in the 19th century by Justus von Liebig. Yeast cells are heated until they rupture, then the cells' own digestive enzymes break their proteins down into simpler compounds (amino acids and peptides), a process called autolysis. The insoluble cell walls are the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inert Gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to as the inert gases. Inert gases are used generally to avoid unwanted chemical reactions degrading a sample. These undesirable chemical reactions are often oxidation and hydrolysis reactions with the oxygen and moisture in air. The term ''inert gas'' is context-dependent because several of the noble gases can be made to react under certain conditions. Purified argon gas is the most commonly used inert gas due to its high natural abundance (78.3% N2, 1% Ar in air) and low relative cost. Unlike noble gases, an inert gas is not necessarily elemental and is often a compound gas. Like the noble gases, the tendency for non-reactivity is due to the valence, the outermost electron shell, being complete in all the inert gases. This is a tendency, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sparging (chemistry)
In chemistry, sparging, also known as gas flushing in metallurgy, is a technique in which a gas is bubbled through a liquid in order to remove ''other'' dissolved gas(es) and/or dissolved volatile liquid(s) from that liquid. It is a method of degassing. According to Henry's law, the concentration of each gas in a liquid is proportional to the partial pressure of that gas (in the gaseous state) in contact with the liquid. Sparging introduces a gas that has little or no partial pressure of the gas(es) to be removed, and increases the area of the gas-liquid interface, which encourages some of the dissolved gas(es) to diffuse into the sparging gas before the sparging gas escapes from the liquid. Many sparging processes, such as solvent removal, use air as the sparging gas. To remove oxygen, or for sensitive solutions or reactive molten metals, a chemically inert gas such as nitrogen, argon, or helium is used. Liquid chromatography Solvents used in high-performance liquid chromatogra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
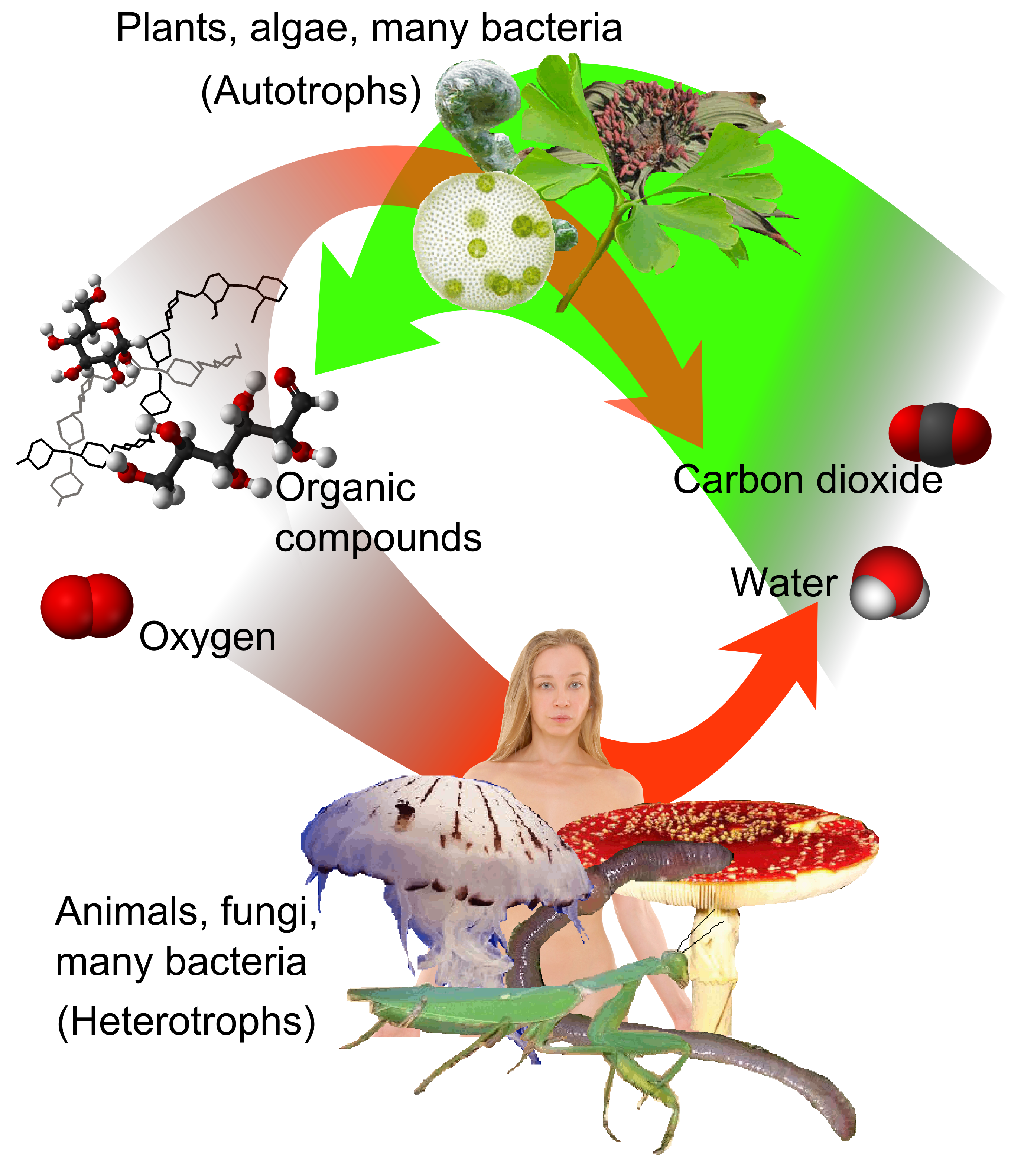
Autotrophic
An autotroph or primary producer is an organism that produces complex organic compounds (such as carbohydrates, fats, and proteins) using carbon from simple substances such as carbon dioxide,Morris, J. et al. (2019). "Biology: How Life Works", 3rd edition, W. H. Freeman. generally using energy from light (photosynthesis) or inorganic chemical reactions (chemosynthesis). They convert an abiotic source of energy (e.g. light) into energy stored in organic compounds, which can be used by other organisms (e.g. heterotrophs). Autotrophs do not need a living source of carbon or energy and are the producers in a food chain, such as plants on land or algae in water (in contrast to heterotrophs as consumers of autotrophs or other heterotrophs). Autotrophs can reduce carbon dioxide to make organic compounds for biosynthesis and as stored chemical fuel. Most autotrophs use water as the reducing agent, but some can use other hydrogen compounds such as hydrogen sulfide. The primary produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, e.g. sodium thiosulfate . Thiosulfate also refers to the esters of thiosulfuric acid, e.g. ''O'',''S''-dimethyl thiosulfate . The prefix thio- indicates that the thiosulfate is a sulfate with one oxygen replaced by sulfur. Thiosulfate is tetrahedral at the central S atom. Thiosulfate salts occur naturally. Thiosulfate ion has C3v symmetry, and is produced by certain biochemical processes. It rapidly dechlorinates water and is notable for its use to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used in dying in textiles and the bleaching of natural substances. Sodium thiosulfate, commonly called ''hypo'' (from "hyposulfite"), was widely used in photography to fix black and white negatives and prints after the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemolithotrophic
Lithotrophs are a diverse group of organisms using an inorganic substrate (usually of mineral origin) to obtain reducing equivalents for use in biosynthesis (e.g., carbon dioxide fixation) or energy conservation (i.e., ATP production) via aerobic or anaerobic respiration. While lithotrophs in the broader sense include photolithotrophs like plants, chemolithotrophs are exclusively microorganisms; no known macrofauna possesses the ability to use inorganic compounds as electron sources. Macrofauna and lithotrophs can form symbiotic relationships, in which case the lithotrophs are called "prokaryotic symbionts". An example of this is chemolithotrophic bacteria in giant tube worms or plastids, which are organelles within plant cells that may have evolved from photolithotrophic cyanobacteria-like organisms. Chemolithotrophs belong to the domains Bacteria and Archaea. The term "lithotroph" was created from the Greek terms 'lithos' (rock) and 'troph' (consumer), meaning "eaters of ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. Fundamentals When dissolved in water, formic acid converts to formate: : Formate is a planar anion. The two oxygen atoms are equivalent and bear a partial negative charge. The remaining C-H bond is not acidic. Biochemistry : Formate is a common C-1 source in living systems. It is formed from many precursors including choline, serine, and sarcosine. It provides a C-1 source in the biosynthesis of some nucleic acids. Formate (or formic acid) is ina leaving group in the demethylation of some sterols.. These conversions are catalyzed by aromatase enzymes using O2 as the oxidant. Specific conversions include testosterone to estradiol and androstenedione to estrone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |