|
Achmatowicz Reaction
The Achmatowicz reaction, also known as the Achmatowicz rearrangement, is an organic synthesis in which a furan is converted to a dihydropyran. In the original publication by the Polish Chemist Osman Achmatowicz Jr. (b. 20 December 1931 in Vilnius) in 1971 furfuryl alcohol is reacted with bromine in methanol to 2,5-dimethoxy-2,5-dihydrofuran which rearranges to the dihydropyran with dilute sulfuric acid. Additional reaction steps, alcohol protection with methyl orthoformate and boron trifluoride) and then ketone reduction with sodium borohydride produce an intermediate from which many monosaccharides can be synthesised. : The Achmatowitz protocol has been used in total synthesis, including those of desoxoprosophylline, pyrenophorin Recently it has been used in diversity oriented synthesis : and in enantiomeric scaffolding.Reagents: benzyl chloroformate protects amine as Cbz group, Achmatowitz reaction with m-CPBA, complexation with a molybdenum compound, Cp is cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Achmatowicz Reaction
''The'' () is a grammatical article in English, denoting persons or things already mentioned, under discussion, implied or otherwise presumed familiar to listeners, readers, or speakers. It is the definite article in English. ''The'' is the most frequently used word in the English language; studies and analyses of texts have found it to account for seven percent of all printed English-language words. It is derived from gendered articles in Old English which combined in Middle English and now has a single form used with pronouns of any gender. The word can be used with both singular and plural nouns, and with a noun that starts with any letter. This is different from many other languages, which have different forms of the definite article for different genders or numbers. Pronunciation In most dialects, "the" is pronounced as (with the voiced dental fricative followed by a schwa) when followed by a consonant sound, and as (homophone of pronoun ''thee'') when followed by a v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Anion
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of and abbreviated as Cp−. It is formed from the deprotonation of the molecule cyclopentadiene. Properties The cyclopentadienyl anion is a planar, cyclic, regular-pentagonal ion; it has 6 Ď€-electrons (4''n'' + 2, where ''n'' = 1), which fulfills HĂĽckel's rule of aromaticity. The structure shown is a composite of five resonance contributors in which each carbon atom carries part of the negative charge. Salt (chemistry), Salts of the cyclopentadienyl anion can be stable, e.g., sodium cyclopentadienide. It can also coordinate as a ligand to metal atoms, forming coordination compounds known as cyclopentadienyl complexes. Biscyclopentadienyl complexes are called metallocenes. Cyclopentadienyl, , and cyclopentadiene, , can substitute one or more hydrogens, forming derivatives having covalent bonds. (See Cyclopentadiene#Derivatives) Abbreviation The abb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. The free element, a silvery metal with a grey cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum compounds have low solubili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
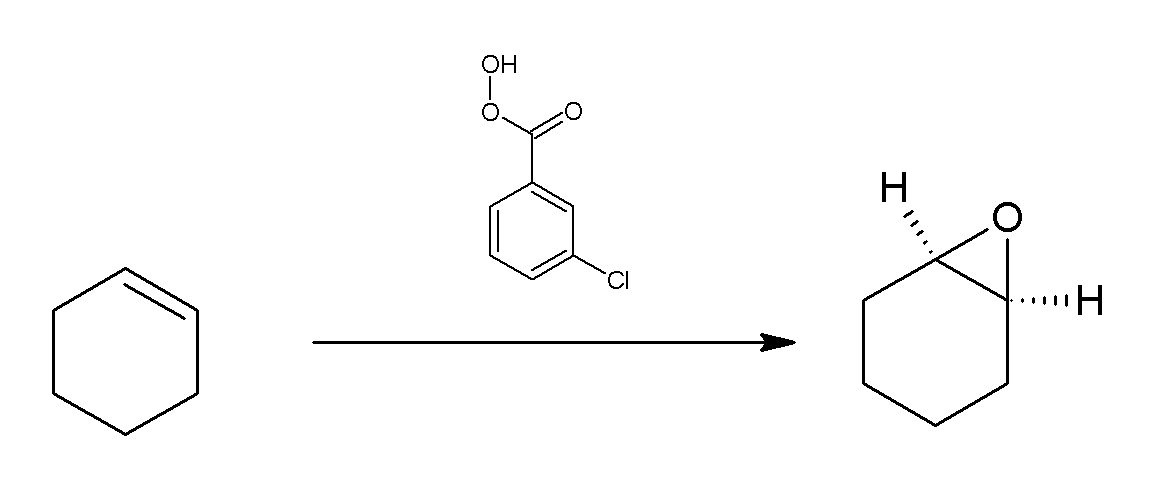
M-CPBA
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. Preparation and purification mCPBA can be prepared by reacting m-Chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification. It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of ''m''-chlorobenzoic acid (10%) and water. The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5. Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so one can extract the acid impurity by careful control of pH. The purified material is reasonably stable against d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Chloroformate
Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl (Cbz or Z) group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water. The compound was first prepared by Leonidas Zervas in the early 1930s who used it for the introduction of the benzyloxycarbonyl protecting group, which became the basis of the Bergmann-Zervas carboxybenzyl method of peptide synthesis he developed with Max Bergmann. This was the first successful method of controlled peptide chemical synthesis and for twenty years it was the dominant procedure used worldwide until the 1950s. To this day, benzyl chloroformate is often used for amine group protection. Preparation The compound is prepared in the lab by treating benzyl alcohol with phosgene: : PhCH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomeric Scaffolding
The chiral pool is a "collection of abundant enantiopure building blocks provided by nature" used in synthesis. In other words, a chiral pool would be a large quantity of common organic enantiomers. Contributors to the chiral pool are amino acids, sugars, and terpenes. Their use improves the efficiency of total synthesis. Not only does the chiral pool contribute a premade carbon skeleton, their chirality is usually preserved in the remainder of the reaction sequence. This strategy is especially helpful if the desired molecule resembles cheap enantiopure natural products. Many times, suitable enantiopure starting materials cannot be identified. The alternative to the use of the chiral pool is asymmetric synthesis, whereby achiral precursors are employed or racemic intermediates are resolved. Examples The use of the chiral pool is illustrated by the synthesis of the anticancer drug paclitaxel (Taxol). The incorporation of the C10 precursor verbenone, a member of the chiral pool, m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid-phase Synthesis
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with normal synthesis in a liquid state include: * High efficiency and throughput * Increased simplicity and speed The reaction can be driven to completion and high yields through the use of excess reagent. In this method, building blocks are protected at all reactive functional groups. The order of functional group reactions can be controlled by the order of deprotection. This method is used for the synthesis of peptides, deoxyribonucleic acid ( DNA), ribonucleic acid (RNA), and other molecules that need to be synthesised in a certain alignment. More recently, this method has also been used in combinatorial chemistry and other synthetic applications. The process was originally developed in the 1950s and 1960s by Robert Bruce Merrifield in orde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridinium Tosylate
Pyridinium ''p''-toluenesulfonate (PPTS) is a colourless solid salt of pyridine and ''p''-toluenesulfonic acid. Uses In organic synthesis, PPTS is used as a weakly acidic catalyst, providing an organic soluble source of pyridinium (C5H5NH+) ions. For example, PPTS is used to deprotect silyl ethers or tetrahydropyranyl ethers when a substrate is unstable to stronger acid catalysts. It is also a commonly used catalyst for the preparation of acetals and ketal In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...s from aldehydes and ketones. References {{Reflist Sulfonates Pyridinium compounds Salts Reagents for organic chemistry P-Tosyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Bromosuccinimide
''N''-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical. Preparation NBS is commercially available. It can also be synthesized in the laboratory. To do so, sodium hydroxide and bromine are added to an ice-water solution of succinimide. The NBS product precipitates and can be collected by filtration. Crude NBS gives better yield in the Wohl-Ziegler reaction. In other cases, impure NBS (slightly yellow in color) may give unreliable results. It can be purified by recrystallization from 90 to 95 °C water (10 g of NBS for 100 mL of water). Reactions Addition to alkenes NBS will react with alkenes 1 in aqueous solvents to give bromohydrins 2. The preferred conditions are the portionwise addition of NBS to a solution of the alkene in 50% aqueous DMSO, DME, THF, or ''tert''-butanol at 0  ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)