|
Acetoxyacetylaminofluorene
Acetoxyacetylaminofluorene is a derivative of 2-acetylaminofluorene used as a biochemical tool in the study of carcinogenesis. It forms adducts with DNA by reacting with guanine at its C-8 position.; This results in breaks in one strand of the DNA. See also * Hydroxyacetylaminofluorene Hydroxyacetylaminofluorene is a derivative of 2-acetylaminofluorene used as a biochemical tool in the study of carcinogenesis Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are ... References {{Organic-compound-stub Carcinogens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-acetylaminofluorene
2-Acetylaminofluorene (AAF, 2-AAF) is a carcinogenic and mutagenic derivative of fluorene. It is used as a biochemical tool in the study of carcinogenesis. It induces tumors in a number of species in the liver, bladder and kidney. The metabolism of this compound in the body by means of biotransformation reactions is the key to its carcinogenicity. 2-AAF is a substrate for cytochrome P-450 (CYP) enzyme, which is a part of a super family found in almost all organisms. This reaction results in the formation of hydroxyacetylaminofluorene which is a proximal carcinogen and is more potent than the parent molecule. The ''N''-hydroxy metabolite undergoes several enzymatic and non-enzymatic rearrangements. It can be O-acetylated by cytosolic N-acetyltransferase enzyme to yield ''N''-acetyl-''N''-acetoxyaminofluorene. This intermediate can spontaneously rearrange to form the arylamidonium ion and a carbonium ion which can interact directly with DNA to produce DNA adducts. In addition to e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyacetylaminofluorene
Hydroxyacetylaminofluorene is a derivative of 2-acetylaminofluorene used as a biochemical tool in the study of carcinogenesis Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abno .... See also * Acetoxyacetylaminofluorene {{organic-compound-stub Carcinogens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
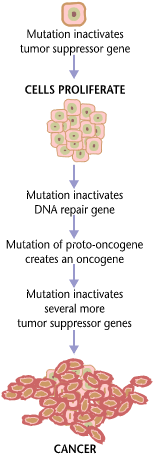
Carcinogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to cancer w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine
Guanine () ( symbol G or Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds. This unsaturated arrangement means the bicyclic molecule is planar. Properties Guanine, along with adenine and cytosine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA, and uracil only in RNA. Guanine has two tautomeric forms, the major keto form (see figures) and rare enol form. It binds to cytosine through three hydrogen bonds. In cytosine, the amino group acts as the hydrogen bond donor and the C-2 carbonyl and the N-3 amine as the hydrogen-bond acceptors. Guanine has the C-6 carbonyl group that acts as the hydrogen bond acceptor, while a group at N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |