|
2-Acetylaminofluorene
2-Acetylaminofluorene (AAF, 2-AAF) is a carcinogenic and mutagenic derivative of fluorene. It is used as a biochemical tool in the study of carcinogenesis. It induces tumors in a number of species in the liver, bladder and kidney. The metabolism of this compound in the body by means of biotransformation reactions is the key to its carcinogenicity. 2-AAF is a substrate for cytochrome P-450 (CYP) enzyme, which is a part of a super family found in almost all organisms. This reaction results in the formation of hydroxyacetylaminofluorene which is a proximal carcinogen and is more potent than the parent molecule. The ''N''-hydroxy metabolite undergoes several enzymatic and non-enzymatic rearrangements. It can be O-acetylated by cytosolic N-acetyltransferase enzyme to yield ''N''-acetyl-''N''-acetoxyaminofluorene. This intermediate can spontaneously rearrange to form the arylamidonium ion and a carbonium ion which can interact directly with DNA to produce DNA adducts. In addition to e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mutagenic
In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in animals, such mutagens can therefore be carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes. The process of DNA becoming modified is called mutagenesis. Not all mutations are caused by mutagens: so-called "spontaneous mutations" occur due to spontaneous hydrolysis, errors in DNA replication, repair and recombination. Discovery The first mutagens to be identified were carcinogens, substances that were shown to be linked to cancer. Tumors were described more than 2,000 years before the discovery of chromosomes and DNA; in 500 B.C., the Greek physician Hippocrates named tumors resembling a crab ''karkinos'' (from which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious. Carcinogens, as mentioned, are agents in the environment capable of contributing to cancer growth. Carcinogens can be categorized into two different types: activation-dependent and activation-independent, and each nature impacts their level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyacetylaminofluorene
Hydroxyacetylaminofluorene is a derivative of 2-acetylaminofluorene used as a biochemical tool in the study of carcinogenesis Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abno .... See also * Acetoxyacetylaminofluorene {{organic-compound-stub Carcinogens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucuronide
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond. The glucuronides belong to the glycosides. Glucuronidation, the conversion of chemical compounds to glucuronides, is a method that animals use to assist in the excretion of toxic substances, drugs or other substances that cannot be used as an energy source. Glucuronic acid is attached via a glycosidic bond to the substance, and the resulting glucuronide, which has a much higher water solubility than the original substance, is eventually excreted by the kidneys. Enzymes that cleave the glycosidic bond of a glucuronide are called glucuronidases. Examples * Miquelianin (Quercetin 3-O-glucuronide) * Morphine-6-glucuronide * Scutellarein-7-glucuronide Scutellarin is a flavone, a type of phenolic chemical compound. It can be found in the plants '' Scutellaria barbata'' and '' S. lateriflora'' which have been used in traditional medicine''.'' T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L- glutamate and cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is catalyzed by glutathione synthetase. While all animal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiols
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strongly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonium
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium (), where the five valences are filled with hydrogen atoms. The next simplest carbonium ions after methanium have two carbon atoms. Ethynium, or protonated acetylene , and ethenium are usually classified in other families. The ethanium ion has been studied as an extremely rarefied gas by infrared spectroscopy. The isomers of octonium (protonated octane, ) have been studied. The carbonium ion has a planar geometry. In older literature, the name "carbonium ion" was used for what is today called carbenium ion, carbenium. The current definitions were proposed by the chemist George Andrew Olah in 1972 and are now widely accepted. A stable carbonium ion is the complex pentakis(triphenylphosphinegold(I))methanium , produced by Schmidbauer and others. Preparation Carbonium ions can be obtained by treating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoxyacetylaminofluorene
Acetoxyacetylaminofluorene is a derivative of 2-acetylaminofluorene used as a biochemical tool in the study of carcinogenesis. It forms adducts with DNA by reacting with guanine at its C-8 position.; This results in breaks in one strand of the DNA. See also * Hydroxyacetylaminofluorene Hydroxyacetylaminofluorene is a derivative of 2-acetylaminofluorene used as a biochemical tool in the study of carcinogenesis Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are ... References {{Organic-compound-stub Carcinogens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
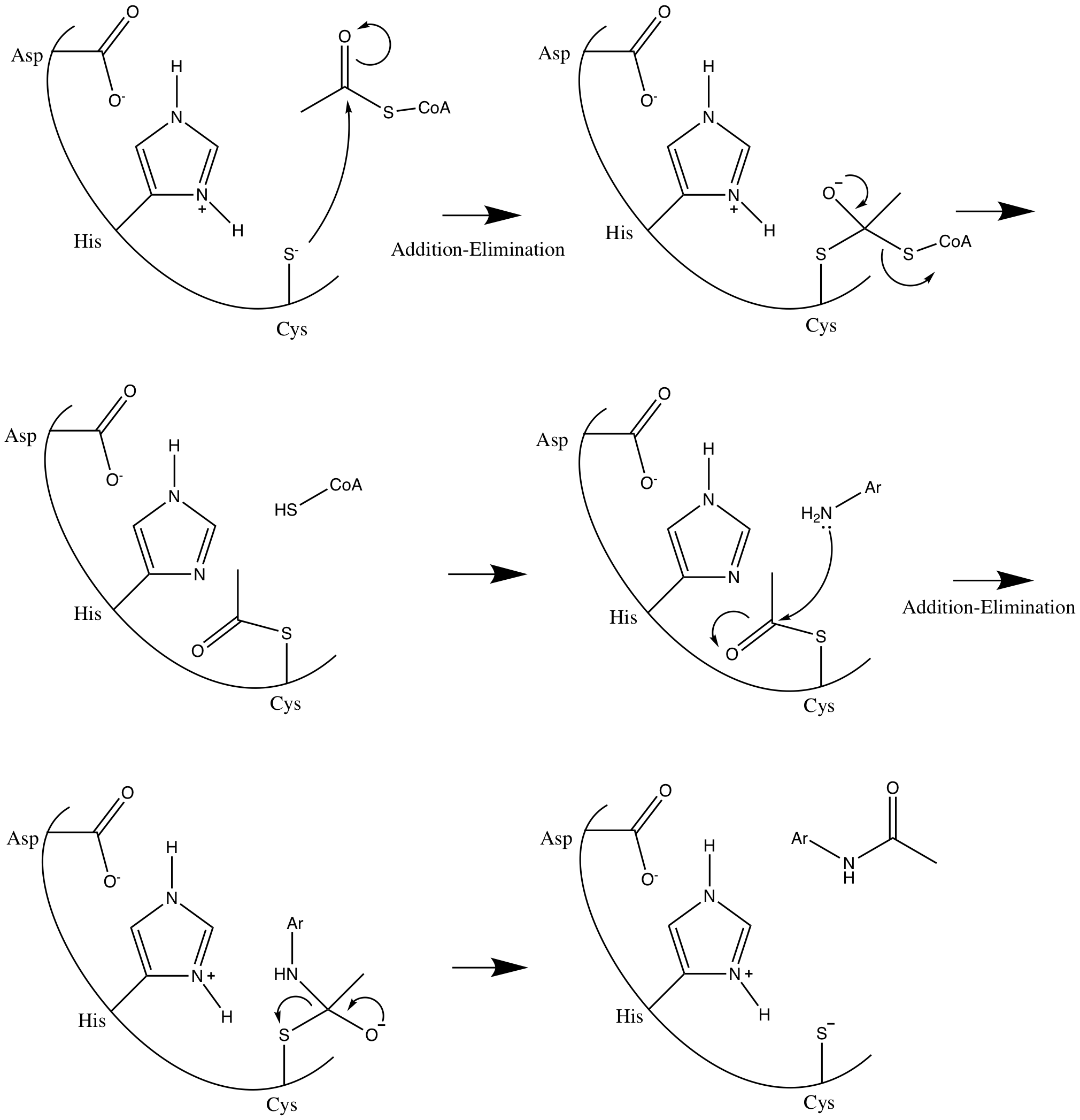
N-acetyltransferase
N-acetyltransferase (NAT) is an enzyme that catalyzes the transfer of acetyl groups from acetyl-CoA to arylamines, arylhydroxylamines and arylhydrazines. They have wide specificity for aromatic amines, particularly serotonin, and can also catalyze acetyl transfer between arylamines without CoA. N-acetyltransferases are cytosolic enzymes found in the liver and many tissues of most mammalian species, except the dog and fox, which cannot acetylate xenobiotics. Acetyl groups are important in the conjugation of metabolites from the liver, to allow excretion of the byproducts (phase II metabolism). This is especially important in the metabolism and excretion of drug products (drug metabolism). __TOC__ Enzyme Mechanism NAT enzymes are differentiated by the presence of a conserved catalytic triad that favors aromatic amine and hydrazine substrates. NATs catalyze the acetylation of small molecules through a double displacement reaction called the ping pong bi bi reaction. The mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |