|
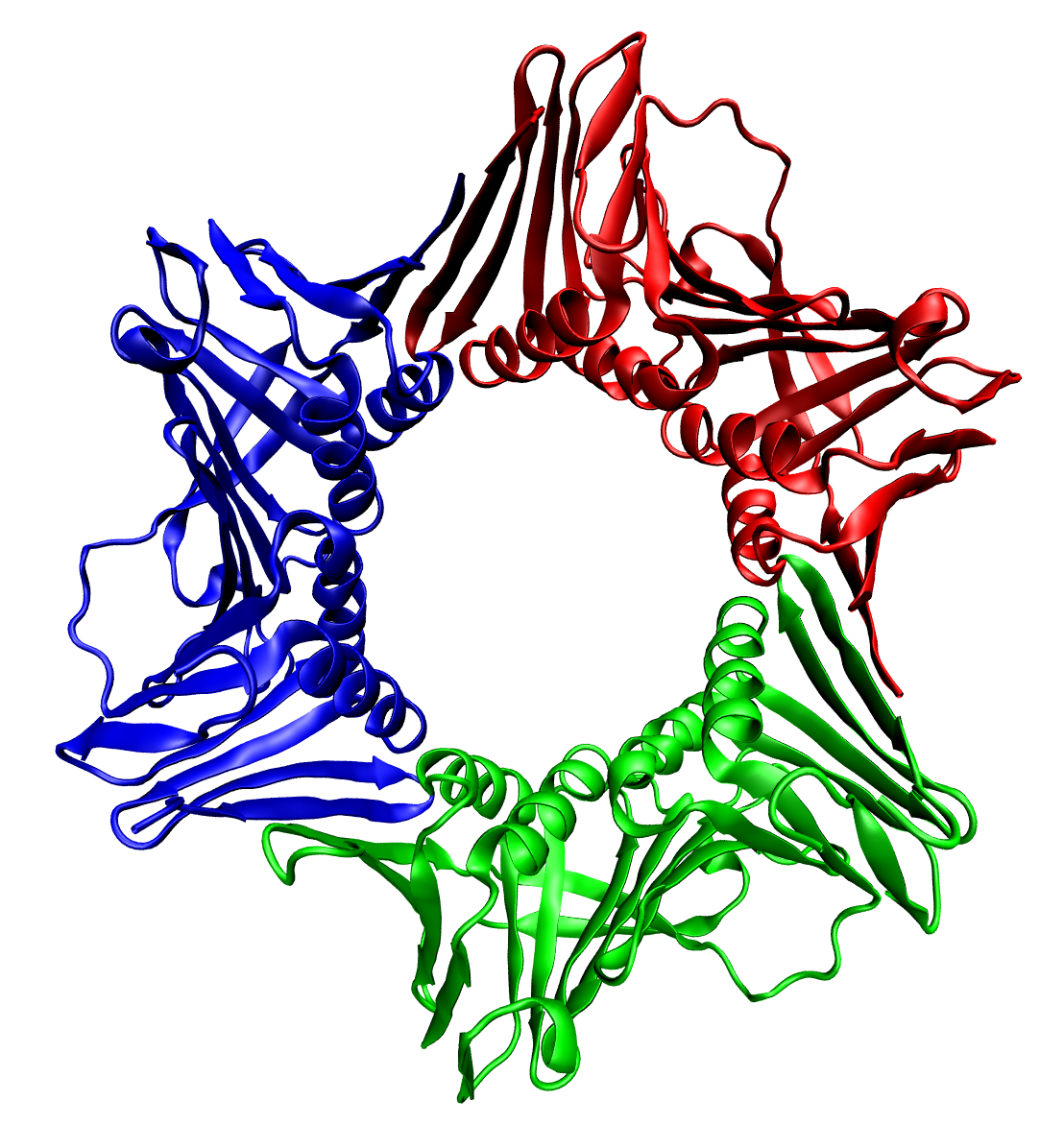
AMP-activated Protein Kinase
5' AMP-activated protein kinase or AMPK or 5' adenosine monophosphate-activated protein kinase is an enzyme (EC 2.7.11.31) that plays a role in cellular energy homeostasis, largely to activate glucose and fatty acid uptake and oxidation when cellular energy is low. It belongs to a highly conserved eukaryotic protein family and its orthologues are SNF1 in yeast, and SnRK1 in plants. It consists of three proteins ( subunits) that together make a functional enzyme, conserved from yeast to humans. It is expressed in a number of tissues, including the liver, brain, and skeletal muscle. In response to binding AMP and ADP, the net effect of AMPK activation is stimulation of hepatic fatty acid oxidation, ketogenesis, stimulation of skeletal muscle fatty acid oxidation and glucose uptake, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipogenesis, inhibition of adipocyte lipolysis, and modulation of insulin secretion by pancreatic β ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active AMPK
Active may refer to: Music * ''Active'' (album), a 1992 album by Casiopea * Active Records, a record label Ships * ''Active'' (ship), several commercial ships by that name * HMS ''Active'', the name of various ships of the British Royal Navy * USCS ''Active'', a US Coast Survey ship in commission from 1852 to 1861 * USCGC ''Active'', the name of various ships of the US Coast Guard * USRC ''Active'', the name of various ships of the US Revenue Cutter Service * USS ''Active'', the name of various ships of the US Navy Computers and electronics * Active Enterprises, a defunct video game developer * Sky Active, the brand name for interactive features on Sky Digital available in the UK and Ireland * Active (software), software used for open publishing by Indymedia; see Independent Media Center Sciences * Thermodynamic activity, measure of an effective concentration of a species in a mixture. * Activation, in chemistry the process whereby something is prepared for a su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pancreatic
The pancreas is an Organ (anatomy), organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdominal cavity, abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine gland, i.e. it has both an endocrine and a digestive exocrine function. 99% of the pancreas is exocrine and 1% is endocrine. As an endocrine gland, it functions mostly to regulate blood sugar levels, secreting the hormones insulin, glucagon, somatostatin, and pancreatic polypeptide. As a part of the digestive system, it functions as an exocrine gland secreting pancreatic juice into the duodenum through the pancreatic duct. This juice contains bicarbonate, which neutralizes acid entering the duodenum from the stomach; and digestive enzymes, which break down carbohydrates, proteins, and lipids, fats in food entering the duodenum from the stomach. Inflammation of the pancreas is known as pancreatitis, with common causes including chronic Alc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate groups to an acceptor, nor with phosphatases, which remove phosphate groups (dephosphorylation). The phosphorylation state of a molecule, whet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Upstream (bioprocess)
A bioprocess is a specific process that uses complete living cells or their components (e.g., bacteria, enzymes, chloroplasts) to obtain desired products. Transport of energy and mass is fundamental to many biological and environmental processes. Areas, from food processing (including brewing beer) to thermal design of buildings to biomedical devices, manufacture of monoclonal antibodies to pollution control and global warming, require knowledge of how energy and mass can be transported through materials (momentum, heat transfer, etc.). Cell bioprocessing Cell therapy bioprocessing is a discipline that bridges the fields of cell therapy and bioprocessing (i.e., biopharmaceutical manufacturing), and is a sub-field of bioprocess engineering. The goals of cell therapy bioprocessing are to establish reproducible and robust manufacturing processes for the production of therapeutic cells.Rowley, J.A. Developing Cell Therapy Biomanufacturing Processes, Chem Eng Progress, SBE Stem Cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP : ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Domain
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) and residues that catalyse a reaction of that substrate (catalytic site). Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes. Each active site is evolved to be optimised to bind a particular substrate and catalyse a particular reaction, resulting in high specificity. This specificity is determined by the arrangement of amino acids within the active site and the structure of the substrates. Sometimes enzymes also need to bind with some cofactors to fulfil their function. The active si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Affinity
In chemical physics and physical chemistry, chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds. Chemical affinity can also refer to the tendency of an atom or compound to combine by chemical reaction with atoms or compounds of unlike composition. History Early theories The idea of ''affinity'' is extremely old. Many attempts have been made at identifying its origins. The majority of such attempts, however, except in a general manner, end in futility since "affinities" lie at the basis of all magic, thereby pre-dating science. Physical chemistry, however, was one of the first branches of science to study and formulate a "theory of affinity". The name ''affinitas'' was first used in the sense of chemical relation by German philosopher Albertus Magnus near the year 1250. Later, those as Robert Boyle, John Mayow, Johann Glauber, Isaac Newton, and Georg Stahl put forward ideas on elective affinity in attempts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar (ribose), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CBS Domain
In molecular biology, the CBS domain is a protein domain found in a range of proteins in all species from bacteria to humans. It was first identified as a conserved sequence region in 1997 and named after cystathionine beta synthase, one of the proteins it is found in. CBS domains are also found in a wide variety of other proteins such as inosine monophosphate dehydrogenase, voltage gated chloride channels and AMP-activated protein kinase (AMPK). CBS domains regulate the activity of associated enzymatic and transporter domains in response to binding molecules with adenosyl groups such as AMP and ATP, or s-adenosylmethionine. Structure The CBS domain is composed of a beta-alpha-beta-beta-alpha secondary structure pattern that is folded into a globular tertiary structure that contains a three-stranded antiparallel β-sheet with two α-helices on one side. CBS domains are always found in pairs in protein sequences and each pair of these domains tightly associate in a pseudo dim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multienzyme complexes, in which multiple catalytic domains are found in a single polypeptide chain. Protein complexes are a form of quaternary structure. Proteins in a protein complex are linked by non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining the components of a complex. Examples of protein complexes include the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotrimeric
In biochemistry, a protein trimer is a macromolecular Complex (chemistry), complex formed by three, usually covalent bond, non-covalently bound, macromolecules like proteins or nucleic acids. A homotrimer would be formed by three identical molecules. A heterotrimer would be formed by three different macromolecules. Type II Collagen is an example of homotrimeric protein. Porin (protein), Porins usually arrange themselves in membranes as trimers. Bacteriophage T4 tail fiber Multiple copies of a polypeptide encoded by a gene often can form an aggregate referred to as a multimer. When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. When a mixed multimer displays increased functionality relative to the unmixed multimers, the phenomenon is referred to as complementation (genetics), intragenic complementation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |