|
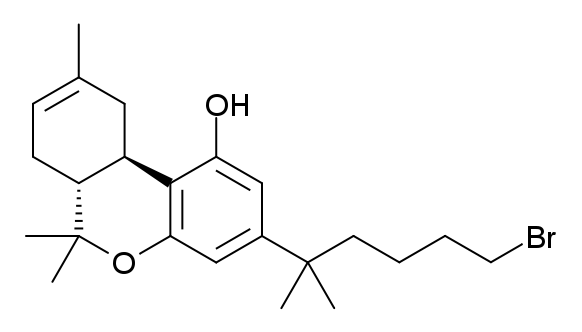
AM-1248
AM-1248 is a drug that acts as a moderately potent agonist for both the cannabinoid receptors CB1 and CB2, but with some dispute between sources over its exact potency and selectivity. Replacing the 3-(1-naphthoyl) group found in many indole derived cannabinoid ligands, with an adamant oyl group, generally confers significant CB2 selectivity, but reasonable CB1 affinity and selectivity is retained when an N-methylpiperidin-2-ylmethyl substitution is used at the indole 1-position. The related compound 1-pentyl-3-(1-adamantoyl)indole was identified as having been sold as a cannabinoid designer drug A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Des ... in Hungary in 2011, along with another synthetic cannabinoid AM-679 (cannabinoid), AM-679. Legality Sweden's public health agency sugg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally ... References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AB-001
AB-001 (1-pentyl-3-(1-adamantoyl)indole) is a designer drug that was found as an ingredient in synthetic cannabis smoking blends in Ireland in 2010 and Hungary and Germany in 2011. It is unclear who AB-001 was originally developed by, but it is structurally related to compounds such as AM-1248 and its corresponding 1-(tetrahydropyran-4-ylmethyl) analogue, which are known to be potent cannabinoid agonists with moderate to high selectivity for CB2 over CB1. The first published synthesis and pharmacological evaluation of AB-001 revealed that it acts as a full agonist at CB1 (EC50 = 35 nM) and CB2 receptors (EC50 = 48 nM). However, AB-001 was found to possess only weak cannabimimetic effects in rats at doses up to 30 mg/kg, making it less potent than the carboxamide analogue APICA, which possesses potent cannabimimetic activity at doses of 3 mg/kg. See also * A-834,735 * AB-005 * AM-1248 * APICA APICA or Apica may refer to: Toponyms * Apica River, stream in Char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A-834,735
A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a ''K''i of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids, with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds, imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (''N''-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall. Legal status As of October 2015 A-834,735 is a controlled substance in China. See also * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabipiperidiethanone
Cannabipiperidiethanone (CPE or 1-(N-methylpiperidin-2-ylmethyl)-3-(2-methoxyphenylacetyl)indole) is a synthetic cannabinoid that has been found as an ingredient of "herbal" synthetic cannabis blends sold in Japan, alongside JWH-122 and JWH-081. Its binding affinity was measured at the CB1 and CB2 receptors and it was found to have an IC50 of 591 nM at CB1 and 968 nM at CB2, making it 2.3 times and 9.4 times weaker than JWH-250 at these two targets respectively. In the United States, CB1 receptor agonists of the 3-phenylacetylindole class such as cannabipiperidiethanone are Schedule I Controlled Substances. See also * AM-1220 * AM-1248 * AM-2233 * JWH-203 JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at ... * RCS-8 References External linksWater Soluble CBD Oil [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1220
AM-1220 is a drug that acts as a potent and moderately selective agonist for the cannabinoid receptor CB1, with around 19 times selectivity for CB1 over the related CB2 receptor. It was originally invented in the early 1990s by a team led by Thomas D'Ambra at Sterling Winthrop, but has subsequently been researched by many others, most notably the team led by Alexandros Makriyannis at the University of Connecticut. The (piperidin-2-yl)methyl side chain of AM-1220 contains a stereocenter, so there are two enantiomers with quite different potency, the (''R'')-enantiomer having a Ki of 0.27 nM at CB1 while the (''S'')-enantiomer has a much weaker Ki of 217 nM. Related compounds A number of related compounds are known with similar potent cannabinoid activity, with modifications such as substitution of the indole ring at the 2- or 6-positions, the naphthoyl ring substituted at the 4-position or replaced by substituted benzoyl rings or other groups, or the 1-(N-methylpiperi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-411
AM-411 (part of the AM cannabinoid series) is an analgesic drug that is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with an adamantyl group at the 3-position, demonstrating that the binding pocket for the alkyl chain at this position can accommodate significant bulk. AM-411 is a potent and fairly selective CB1 full agonist with a Ki of 6.80 nM, but is still also a moderately potent CB2 agonist with a Ki of 52.0 nM. It produces similar effects to other cannabinoid agonists such as analgesia, sedation, and anxiolysis. See also * AM-087 * AM-1248 * KM-233 KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of ''Cannabis''. KM-233 differs from Δ8-THC by the pentyl side chain being r ... References Adamantanes Benzochromenes Phenols AM cannabinoids {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoalkylindoles
Aminoalkylindoles (AAIs) are a family of cannabinergic compound that act as a cannabinoid receptor agonist. They were invented by pharmaceutical company Sterling-Winthrop in the early 1990s as potential nonsteroidal anti-inflammatory agents. Legality Aminoalkylindoles are now commonly found in synthetic cannabis designer drugs. In the United States, the DEA added the aminoalkylindoles JWH-200 to Schedule I of the Controlled Substances Act on 1 March 2011 for 12 months. References {{reflist External links Aminoalkylindoles ChEBI Chemical Entities of Biological Interest, also known as ChEBI, is a chemical database and ontology of molecular entities focused on 'small' chemical compounds, that is part of the Open Biomedical Ontologies (OBO) effort at the European Bioinform ... ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-2233
AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a ''K''i of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (''R'') enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2. It is notable for inducing tinnitus, though the reasons for this are unclear and may provide valuable insight into tinnitus research. Legal Status As of October 2015 AM-2233 is a controlled substance in China. See also * AM-679 * AM-694 * AM-1220 * AM-1221 * AM-1235 * AM-1241 * AM-2232 * Cannabipiperidiethanone * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of designer drugs Designer drugs are structural or functional analogues of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-679 (cannabinoid)
AM-679 (part of the AM cannabinoid series) is a drug that acts as a moderately potent agonist for the cannabinoid receptors, with a ''K''i of 13.5 nM at CB1 and 49.5 nM at CB2. AM-679 was one of the first 3-(2-iodobenzoyl)indole derivatives that was found to have significant cannabinoid receptor affinity, and while AM-679 itself has only modest affinity for these receptors, it was subsequently used as a base to develop several more specialised cannabinoid ligands that are now widely used in research, including the potent CB1 agonists AM-694 and AM-2233, and the selective CB2 agonist AM-1241. AM-679 was first identified as having been sold as a cannabinoid designer drug in Hungary in 2011, along with another novel compound 1-pentyl-3-(1-adamantoyl)indole. See also * RCS-4 RCS-4, or 1-pentyl-3-(4-methoxybenzoyl)indole, is a synthetic cannabinoid drug sold under the names SR-19, BTM-4, or Eric-4 (later shortened to E-4), but originally, OBT-199. Pharmacology RCS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
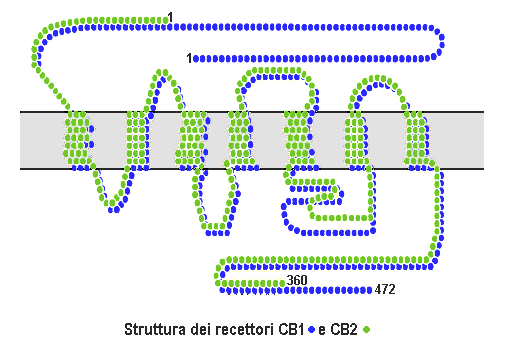
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |