|
ACOT11
Acyl-coenzyme A thioesterase 11 also known as StAR-related lipid transfer protein 14 (STARD14) is an enzyme that in humans is encoded by the ''ACOT11'' gene. This gene encodes a protein with acyl-CoA thioesterase activity towards medium (C12) and long-chain (C18) fatty acyl-CoA substrates which relies on its StAR-related lipid transfer domain. Expression of a similar murine protein in brown adipose tissue is induced by cold exposure and repressed by warmth. Expression of the mouse protein has been associated with obesity, with higher expression found in obesity-resistant mice compared with obesity-prone mice. Alternative splicing results in two transcript variants encoding different isoforms. Structure The ACOT11 gene is located on the 1st chromosome, with its specific localization being 1p32.3. It contains 18 exons. The protein encoded by this gene contains 258 amino acids, and forms a homodimer with another chain. Its theoretical weight is 26.67 kDa. The protein contains a St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioesterase
Thioesterases are enzymes which belong to the esterase family. Esterases, in turn, are one type of the several hydrolases known. Thioesterases exhibit esterase activity (splitting of an ester into acid and Alcohol (chemistry), alcohol, in the presence of water) specifically at a thiol group. Thioesterases or thiolester hydrolases are identified as members of EC 3.1.2. Family The thioesterase activity is performed by members of the acyl-CoA thioesterase (ACOT) family. The regulatory role of ACOT in fatty acid metabolism depends on their substrate (biology), substrate specificity, tissue expression and subcellular localization. For example, deactivation of fatty acids at the ER may traffic fatty acids away from pathways associated with the ER membrane, such as glycerolipid biosynthesis. Two structurally different ACOT types lead to a similar enzymatic activity in vitro, dividing the family into type I and type II ACOTs. Type I ACOTs (ACOT1–6) contain the α/β-hydrolase domain, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
StAR-related Transfer Domain
START (StAR-related lipid-transfer) is a lipid-binding domain in StAR, HD-ZIP and signalling proteins. The archetypical domain is found in StAR ( Steroidogenic acute regulatory protein), a mitochondrial protein that is synthesized in steroid-producing cells. StAR initiates steroid production by mediating the delivery of cholesterol to the first enzyme in steroidogenic pathway. The START domain is critical for this activity, perhaps through the binding of cholesterol. Following the discovery of StAR, 15 START-domain-containing proteins (termed STARD1 through STARD15) were subsequently identified in vertebrates as well as other that are related. Thousands of proteins containing at least one START domain have been determined in invertebrates, bacteria and plants to form a larger superfamily, variously known as START, Bet v1-like or SRPBCC (START/RHOalphaC/ PITP/Bet v1/CoxG/CalC) domain proteins, all of which bind hydrophobic ligands. In the case of plants, many of the START pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
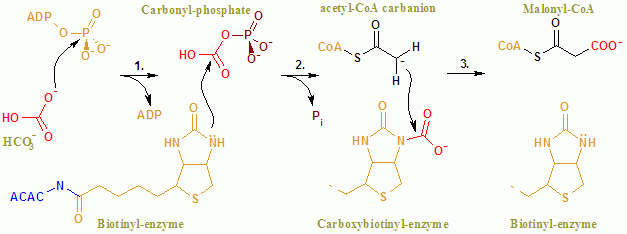
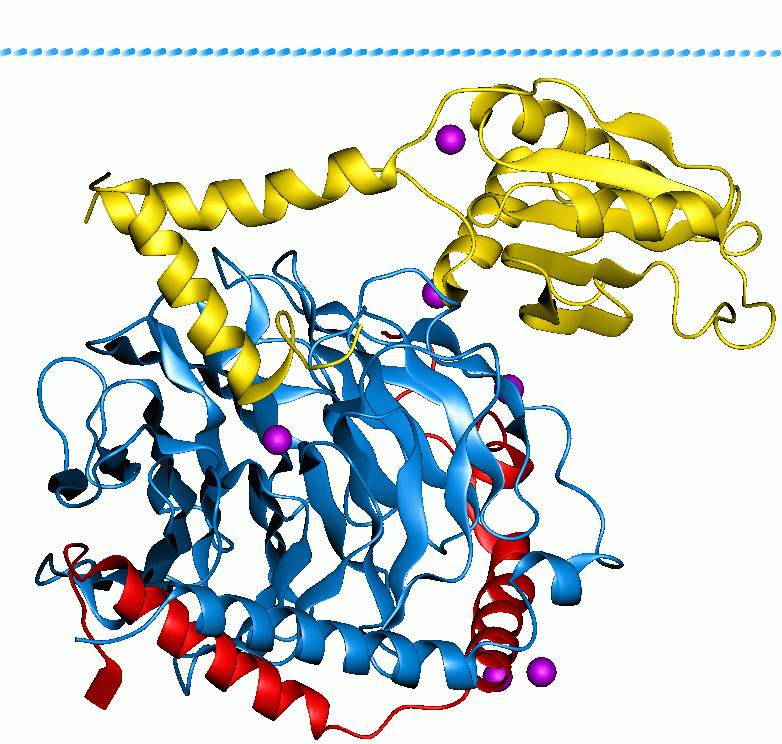
Acetyl-CoA Carboxylase
Acetyl-CoA carboxylase (ACC) is a biotin-dependent enzyme () that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase (BC) and carboxyltransferase (CT). ACC is a multi-subunit enzyme in most prokaryotes and in the chloroplasts of most plants and algae, whereas it is a large, multi-domain enzyme in the cytoplasm of most eukaryotes. The most important function of ACC is to provide the malonyl-CoA substrate for the biosynthesis of fatty acids. The activity of ACC can be controlled at the transcriptional level as well as by small molecule modulators and covalent modification. The human genome contains the genes for two different ACCs—'' ACACA'' and ''ACACB''. Structure Prokaryotes and plants have multi-subunit ACCs composed of several polypeptides. Biotin carboxylase (BC) activity, biotin carboxyl carrier protein (BCCP), and carboxyl transferase (CT) activity are each contained on a different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The cofactor is, therefore, found in two forms in cells: NAD is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydrogenase
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde. IUBMB Classification Oxidoreductases, enzymes that catalyze oxidation-reduction reactions, constitute Class EC 1 of the IUBMB classification of enzyme-catalyzed reactions. Any of these may be called dehydrogenases, especially those in which NAD+ is the electron acceptor (oxidant), but reductase is also used when the physiological emphasis on reduction of the substrate, and oxidase is used ''only'' when O2 is the electron acceptor. The systematic name of an oxidoreductase is "donor:acceptor oxidore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondria
A mitochondrion (; ) is an organelle found in the Cell (biology), cells of most Eukaryotes, such as animals, plants and Fungus, fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into mitosome, other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitocho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of '' alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are activ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
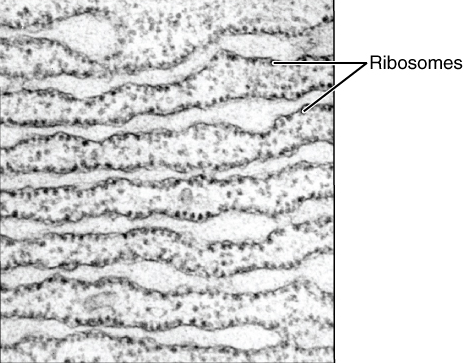
Endomembrane System
The endomembrane system is composed of the different membranes (endomembranes) that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that forms a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of plastids or mitochondria, but might have evolved partially from the actions of the latter (see below). The nuclear membrane contains a lipid bilayer that encompasses the contents of the nucleus. The endoplasmic reticulum (ER) is a synthesis and transport organelle that branches int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinoic Acid
Retinoic acid (used simplified here for all-''trans''-retinoic acid) is a metabolite of vitamin A1 (all-''trans''-retinol) that mediates the functions of vitamin A1 required for growth and development. All-''trans''-retinoic acid is required in chordate animals, which includes all higher animals from fish to humans. During early embryonic development, all-''trans''-retinoic acid generated in a specific region of the embryo helps determine position along the embryonic anterior/posterior axis by serving as an intercellular signaling molecule that guides development of the posterior portion of the embryo. It acts through Hox genes, which ultimately control anterior/posterior patterning in early developmental stages. All-''trans''-retinoic acid (ATRA) is the major occurring retinoic acid, while isomers like 13-''cis''- and 9-''cis''-retinoic acid are also present in much lower levels. The key role of all-''trans''-retinoic acid in embryonic development mediates the high teratogenici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase C
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades. In biochemistry, the PKC family consists of fifteen isozymes in humans. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, DAG, and a phospholipid such as phosphatidylserine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |