|
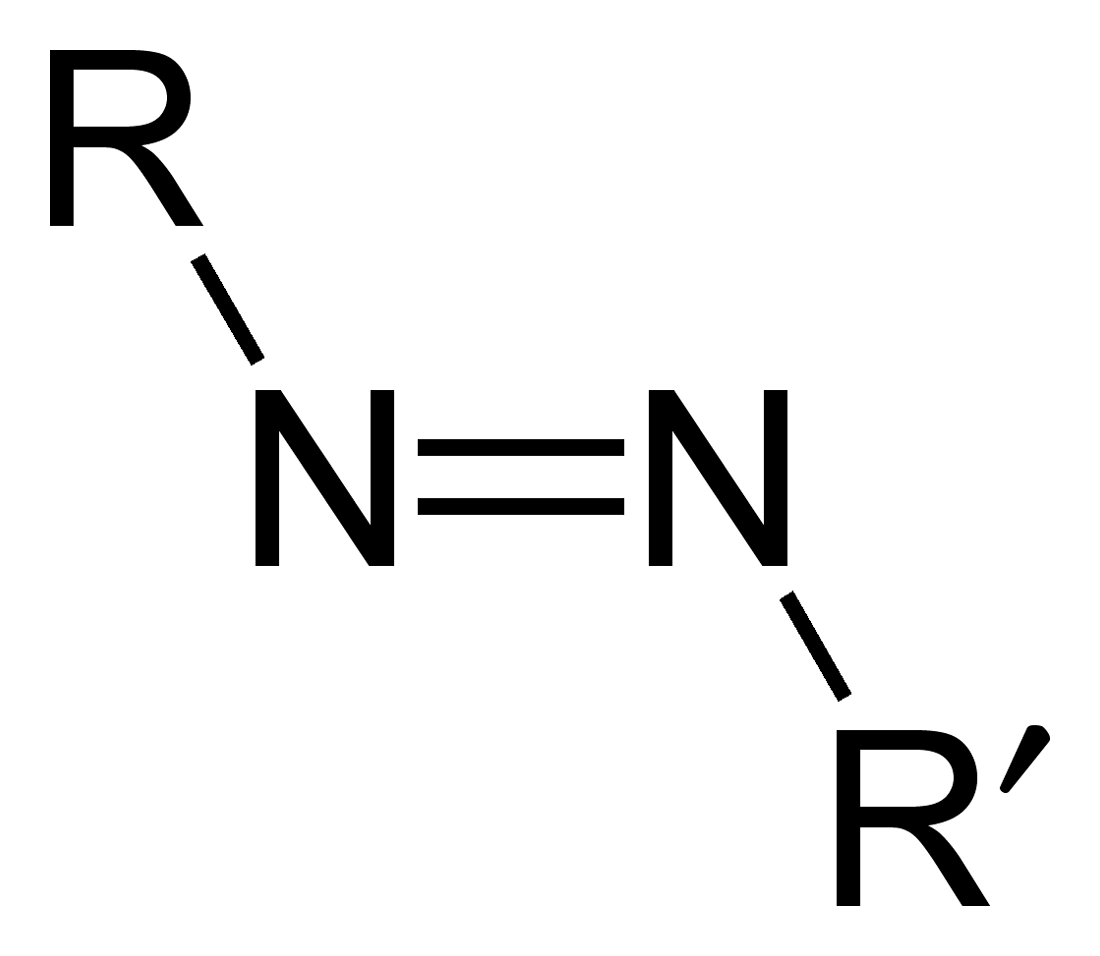
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acene
In organic chemistry, the acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of benzene () rings which have been linearly fused. They follow the general molecular formula . The larger representatives have potential interest in optoelectronic applications and are actively researched in chemistry and electrical engineering. Pentacene has been incorporated into organic field-effect transistors, reaching charge carrier mobilities as high as 5 cm2/Vs. The first 5 unsubstituted members are listed in the following table: Hexacene is not stable in air, and dimerises upon isolation. Heptacene (and larger acenes) is very reactive and has only been isolated in a matrix. However, bis(trialkylsilylethynylated) versions of heptacene have been isolated as crystalline solids. Larger acenes Due to their increased conjugation length the larger acenes are also studied. Theoretically, a number of reports are available on longer chains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Coupling
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic. Diazotization The process of conversion of primary aromatic amines into its diazonium salt is called diazotization. Diazonium salts are important synthetic intermediates that can undergo coupling reactions to form azo dyes and electrophilic substitution reactions to introduce functional groups. Uses of the reaction Aromatic azo compounds tend to be brightly colored due to the extended conjugated systems. Many are used as dyes (see azo dye). Important azo dyes include methyl red and pigment red 170. Azo printing exploits this reaction as well. Azo coupling is also used to produce prontosil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD-R
CD-R (Compact disc-recordable) is a digital optical disc storage format. A CD-R disc is a compact disc that can be written once and read arbitrarily many times. CD-R discs (CD-Rs) are readable by most CD readers manufactured prior to the introduction of CD-R, unlike CD-RW discs. History Originally named CD Write-Once (WO), the CD-R specification was first published in 1988 by Philips and Sony in the Orange Book, which consists of several parts that provide details of the CD-WO, CD-MO (Magneto-Optic), and later CD-RW (ReWritable). The latest editions have abandoned the use of the term "CD-WO" in favor of "CD-R", while " CD-MO" was rarely used. Written CD-Rs and CD-RWs are, in the aspect of low-level encoding and data format, fully compatible with the audio CD (''Red Book'' CD-DA) and data CD (''Yellow Book'' CD-ROM) standards. The Yellow Book standard for CD-ROM only specifies a high-level data format and refers to the Red Book for all physical format and low-level code ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DVD+R
DVD recordable and DVD rewritable are optical disc recording technologies. Both terms describe DVD optical discs that can be written to by a DVD recorder, whereas only 'rewritable' discs are able to erase and rewrite data. Data is written ('burned') to the disc by a laser, rather than the data being 'pressed' onto the disc during manufacture, like a DVD-ROM. Pressing is used in mass production, primarily for the distribution of home video. Like CD-Rs, DVD recordable uses dye to store the data. During the burning of a single bit, the laser's intensity affects the reflective properties of the burned dye. By varying the laser intensity quickly, high density data is written in precise tracks. Since written tracks are made of darkened dye, the data side of a recordable DVD has a distinct color. Burned DVDs have a higher failure-to-read rate than pressed DVDs, due to differences in the reflective properties of dye compared to the aluminum substrate of pressed discs. Comparing re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Orange
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the p''K''a of a mid strength acid, it is usually used in titration of strong acids in weak bases that reach the equivalence point at a pH of 3.1-4.4. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity. Indicator colors In a solution that decreases in acidity, methyl orange moves from the color red to orange and finally to yellow with the opposite occurring for a solution increasing in acidity. This color change from yellow to red occurs because the protons in the acidic solution react with the N=N b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disperse Orange 1
Disperse Orange 1, or 4-anilino-4'-nitroazobenzene, is an azo dye. Commercial samples contain approximately 25% dye by weight, with the remaining mass consisting of NaCl and other salts. This dye is useful in conducting experiments with flash photolysis due to the isomerization In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautome ... effect between the trans-4A4N and cis-4A4N states that occurs during photo relaxation.Wildes, P. D.; Pacifici, J. G.; Irick, G.; Whitten, D. G. J. Am. Chem. Soc., 1971, 93, 2004. References {{reflist Azo dyes Nitrobenzenes Anilines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref> The term delocalization is general and can have slightly different meanings in different fields: * In organic chemistry, it refers to resonance in conjugated systems and aromatic compounds. * In solid-state physics, it refers to free electrons that facilitate electrical conduction. * In quantum chemistry, it refers to molecular orbital electrons that have extended over several adjacent atoms. Resonance In the simple aromatic ring of benzene, the delocalization of six π electrons over the C6 ring is often graphically indicated by a circle. The fact that the six C-C bonds are equidistant is one indication that the electrons are delocalized; if the structure were to have isolated double bonds alternating with discrete single bonds, the bond would likewise have alternating longer and shorter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disodium 4,4'-dinitrostilbene-2,2'-disulfonate
Disodium 4,4′-dinitrostilbene-2,2′-disulfonate is an organic compound with the formula (O2NC6H3(SO3Na)CH)2. This salt is a common precursor to a variety of textile dyes and optical brighteners Preparation and reactions The synthesis of disodium 4,4′-dinitrostilbene-2,2′-disulfonate begins with sulfonation of 4-nitrotoluene. This reaction affords 4-nitrotoluene-2-sulfonic acid. Oxidation of this species with sodium hypochlorite yields the disodium salt of 4,4′-dinitrostilbene-2,2′-disulfonic acid. The product is useful as its reaction with aniline derivatives results in the formation of azo dyes. Commercially important dyes derived from this compound include Direct Red 76, Direct Brown 78, and Direct Orange 40. Reduction gives 4,4′-diamino-2,2′-stilbenedisulfonic acid, which is a common optical brightener. History Arthur Green and André Wahl first reported the formation of disodium 4,4'-dinitrostilbene-2,2'-disulfonate using sodium hypochlorite.{{cite journa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azoxy
In chemistry, azoxy compounds are a group of chemical compounds sharing a common functional group with the general structure . They are considered N-oxides of azo compounds. Azoxy compounds are 1,3-dipoles. They undergo 1,3-dipolar cycloaddition with double bonds. Preparation Most azoxy-containing compounds have aryl substituents. They are typically prepared by reduction of nitrocompounds, such as the reduction of nitrobenzene with arsenous oxide to azoxybenzene. Such reactions are proposed to proceed via the intermediacy of the hydroxylamine and nitroso compounds, e.g. phenylhydroxylamine and nitrosobenzene (Ph = phenyl In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydroge ..., ): :PhNHOH + PhNO -> PhN(O)NPh + H2O Safety Alkyl azoxy compounds, e.g. azoxymethane are suspec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in- space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |