|
Azlocillin
Azlocillin is an acyl ampicillin antibiotic with an extended spectrum of activity and greater ''in vitro'' potency than the carboxy penicillins. Azlocillin is similar to mezlocillin and piperacillin. It demonstrates antibacterial activity against a broad spectrum of bacteria, including ''Pseudomonas aeruginosa'' and, in contrast to most cephalosporins, exhibits activity against enterococci. Spectrum of bacterial susceptibility Azlocillin is considered a broad spectrum antibiotic and can be used against a number of Gram positive and Gram negative bacteria. The following represents MIC susceptibility data for a few medically significant organisms. * ''Escherichia coli'' 1 μg/mL – 32 μg/mL * ''Haemophilus'' spp. 0.03 μg/mL – 2 μg/mL * ''Pseudomonas aeruginosa'' 4 μg/mL – 6.25 μg/mL Synthesis An interesting alternative synthesis of azlocillin involves activation of the substituted phenylglycine analogue 1 with 1,3-dimethyl-2-chloro-1-imidazolinium chloride (2) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azlocillin Synthesis
Azlocillin is an acyl ampicillin antibiotic with an extended spectrum of activity and greater ''in vitro'' potency than the carboxy penicillins. Azlocillin is similar to mezlocillin and piperacillin. It demonstrates antibacterial activity against a broad spectrum of bacteria, including ''Pseudomonas aeruginosa'' and, in contrast to most cephalosporins, exhibits activity against enterococci. Spectrum of bacterial susceptibility Azlocillin is considered a broad spectrum antibiotic and can be used against a number of Gram positive and Gram negative bacteria. The following represents MIC susceptibility data for a few medically significant organisms. * ''Escherichia coli'' 1 μg/mL – 32 μg/mL * ''Haemophilus'' spp. 0.03 μg/mL – 2 μg/mL * ''Pseudomonas aeruginosa'' 4 μg/mL – 6.25 μg/mL Synthesis An interesting alternative synthesis of azlocillin involves activation of the substituted phenylglycine analogue 1 with 1,3-dimethyl-2-chloro-1-imidazolinium chloride (2) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azlocillin Synthesis2
Azlocillin is an acyl ampicillin antibiotic with an extended spectrum of activity and greater ''in vitro'' potency than the carboxy penicillins. Azlocillin is similar to mezlocillin and piperacillin. It demonstrates antibacterial activity against a broad spectrum of bacteria, including ''Pseudomonas aeruginosa'' and, in contrast to most cephalosporins, exhibits activity against enterococci. Spectrum of bacterial susceptibility Azlocillin is considered a broad spectrum antibiotic and can be used against a number of Gram positive and Gram negative bacteria. The following represents MIC susceptibility data for a few medically significant organisms. * ''Escherichia coli'' 1 μg/mL – 32 μg/mL * ''Haemophilus'' spp. 0.03 μg/mL – 2 μg/mL * ''Pseudomonas aeruginosa'' 4 μg/mL – 6.25 μg/mL Synthesis An interesting alternative synthesis of azlocillin involves activation of the substituted phenylglycine analogue 1 with 1,3-dimethyl-2-chloro-1-imidazolinium chloride (2) a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ampicillin
Ampicillin is an antibiotic used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously. Common side effects include rash, nausea, and diarrhea. It should not be used in people who are allergic to penicillin. Serious side effects may include ''Clostridium difficile'' colitis or anaphylaxis. While usable in those with kidney problems, the dose may need to be decreased. Its use during pregnancy and breastfeeding appears to be generally safe. Ampicillin was discovered in 1958 and came into commercial use in 1961. It is on the World Health Organization's List of Essential Medicines. The World Health Organization classifies ampicillin as critically important for human medicine. It is available as a generic medication. Medical uses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillins
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC name: alkanoyl) is usually derived from a carboxylic acid, in which case it has the formula , where R represents an alkyl group that is linked to the carbon atom of the group by a single bond. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond. Compounds Well-known acyl compounds are the acyl chlorides, such as acetyl chloride (CH3COCl) and benzoyl chloride (C6H5COCl). These compounds, which are treated as sources of acylium cations, are good reagents for attac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methicillin
Methicillin (USAN), also known as meticillin (INN), is a narrow-spectrum β-lactam antibiotic of the penicillin class. Methicillin was discovered in 1960. Medical uses Compared to other penicillins that face antimicrobial resistance due to β-lactamase, it is less active, can be administered only parenterally, and has a higher frequency of interstitial nephritis, an otherwise-rare adverse effect of penicillins. However, selection of methicillin depended on the outcome of susceptibility testing of the sampled infection, and since it is no longer produced, it is also not routinely tested for any more. It also served a purpose in the laboratory to determine the antibiotic sensitivity of ''Staphylococcus aureus'' to other penicillins facing β-lactam resistance; this role has now been passed on to other penicillins, namely ''cloxacillin'', as well as genetic testing for the presence of ''mecA'' gene by '' PCR''. Spectrum of activity At one time, methicillin was used to tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-APA
6-APA ((+)-6-aminopenicillanic acid) is a chemical compound used as an intermediate in the synthesis of β-lactam antibiotics. The major commercial source of 6-APA is still natural penicillin G: the semi-synthetic penicillins derived from 6-APA are also referred to as penicillins and are considered part of the penicillin family of antibiotics. In 1958, Beecham scientists from Brockham Park, Surrey, found a way to obtain 6-APA from penicillin. Other β-lactam antibiotics could then be synthesized by attaching various side-chains to the nucleus.F.P. Doyle, J.H.C. Nayler, G.N. Rolinson ''US Patent 2,941,995'', filed July 22, 1958, granted June 21, 1960. Recovery of solid 6-aminopenicillanic acid. The reason why this was achieved so many years after the commercial development of penicillin by Howard Florey and Ernst Chain lies in the fact that penicillin itself is very susceptible to hydrolysis Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylglycine
Phenylglycine is the organic compound with the formula C6H5CH(NH2)CO2H. It is a non-proteinogenic alpha amino acid related to alanine, but with a phenyl group in place of the methyl group. It is a white solid. The compound exhibits some biological activity. Preparation It is prepared from benzaldehyde by amino cyanation (Strecker synthesis). It can also be prepared from glyoxal and by reductive amination Reductive amination (also known as reductive alkylation) is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered ... of phenylglyoxylic acid. Ester The ester methyl α‐phenylglycinate is used to convert carboxylic acids into homologated unsaturated ketones. These reactions proceed via cyclization of phenylglycinamides to oxazolones, which can be reductively cleaved with chromous reagents.{{cite encyclopedia , title=Methyl α‐Phenylgl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cephalosporin
The cephalosporins (sg. ) are a class of β-lactam antibiotics originally derived from the fungus ''Acremonium'', which was previously known as ''Cephalosporium''. Together with cephamycins, they constitute a subgroup of β-lactam antibiotics called cephems. Cephalosporins were discovered in 1945, and first sold in 1964. Discovery The aerobic mold which yielded cephalosporin C was found in the sea near a sewage outfall in Su Siccu, by Cagliari harbour in Sardinia, by the Italian pharmacologist Giuseppe Brotzu in July 1945. Structure Cephalosporin contains a 6-membered dihydrothiazine ring. Substitutions at position 3 generally affect pharmacology; substitutions at position 7 affect antibacterial activity, but these cases are not always true. Medical uses Cephalosporins can be indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic. First-generation cephalosporins are active predominantly against Gram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enterococcus
''Enterococcus'' is a large genus of lactic acid bacteria of the phylum Bacillota. Enterococci are gram-positive cocci that often occur in pairs (diplococci) or short chains, and are difficult to distinguish from streptococci on physical characteristics alone. Two species are common commensal organisms in the intestines of humans: '' E. faecalis'' (90–95%) and '' E. faecium'' (5–10%). Rare clusters of infections occur with other species, including ''E. casseliflavus'', '' E. gallinarum'', and ''E. raffinosus''. Physiology and classification Enterococci are facultative anaerobic organisms, i.e., they are capable of cellular respiration in both oxygen-rich and oxygen-poor environments. Though they are not capable of forming spores, enterococci are tolerant of a wide range of environmental conditions: extreme temperature (10–45 °C), pH (4.6–9.9), and high sodium chloride concentrations. Enterococci typically exhibit gamma-hemolysis on sheep's blood agar. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
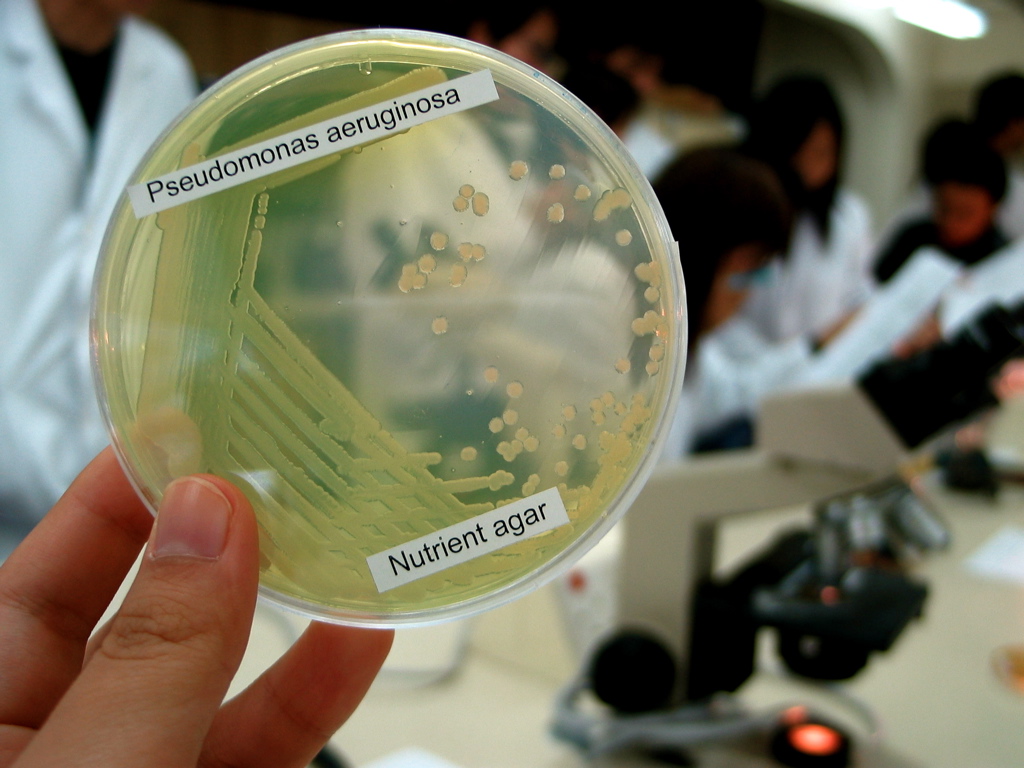
Pseudomonas Aeruginosa
''Pseudomonas aeruginosa'' is a common encapsulated, gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, ''P. aeruginosa'' is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. The organism is considered opportunistic insofar as serious infection often occurs during existing diseases or conditions – most notably cystic fibrosis and traumatic burns. It generally affects the immunocompromised but can also infect the immunocompetent as in hot tub folliculitis. Treatment of ''P. aeruginosa'' infections can be difficult due to its natural resistance to antibiotics. When more advanced antibiotic drug regimens are needed adverse effects may re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |