|
Azane People
Azanes are acyclic, saturated hydronitrogens, which means that they consist only of hydrogen and nitrogen atoms and all bonds are single bonds. They are therefore pnictogen hydrides. Because cyclic hydronitrogens are excluded by definition, the azanes comprise a homologous series of inorganic compounds with the general chemical formula . Each nitrogen atom has three bonds (either N-H or N-N bonds), and each hydrogen atom is joined to a nitrogen atom (H-N bonds). A series of linked nitrogen atoms is known as the nitrogen skeleton or nitrogen backbone. The number of nitrogen atoms is used to define the size of the azane (e.g. N2-azane). The simplest possible azane (the parent molecule) is ammonia, . There is no limit to the number of nitrogen atoms that can be linked together, the only limitation being that the molecule is acyclic, is saturated, and is a hydronitrogen. Azanes are reactive and have significant biological activity. Azanes can be viewed as a more biologically act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azene
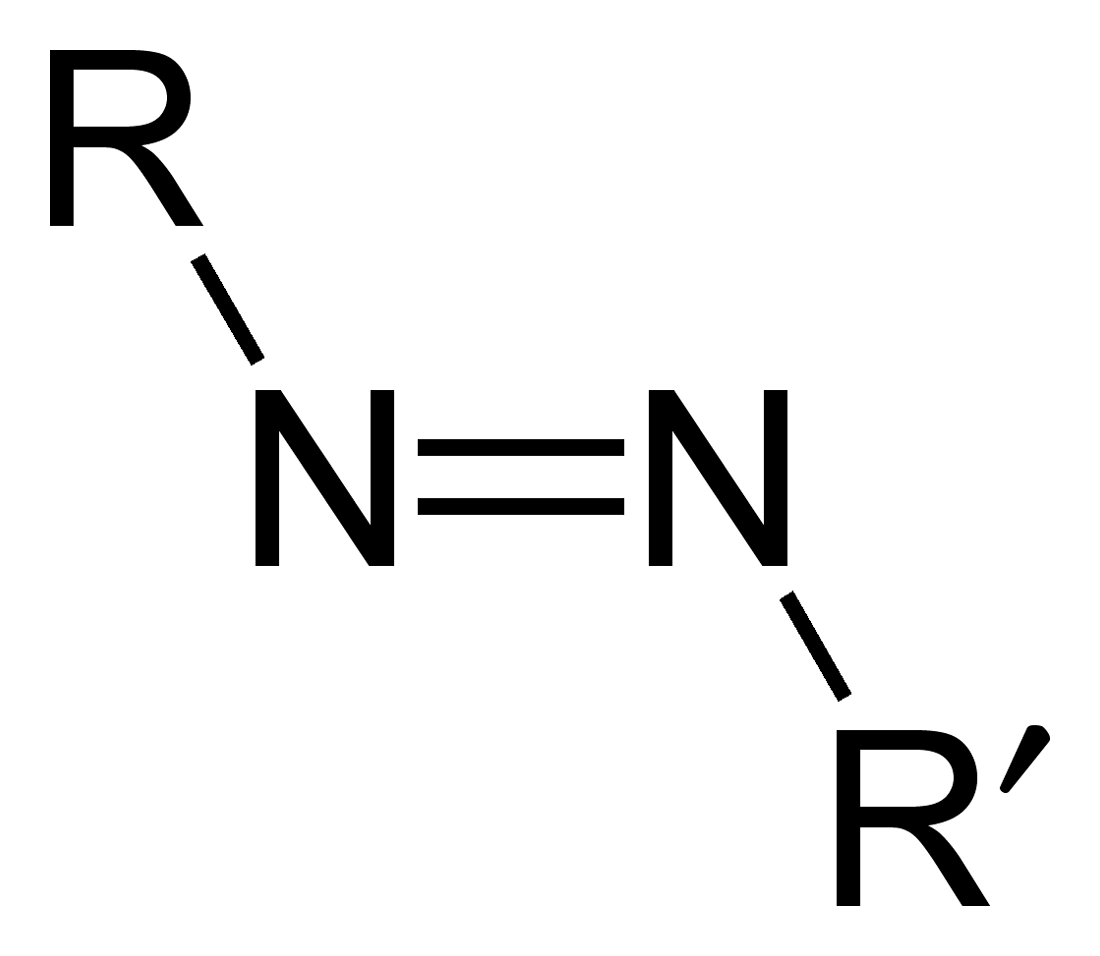
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an diazonium salt, aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a chemical compound, compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct chemical bond, bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azanyl
In chemistry, the amino radical, , also known as the aminyl radical or azanyl radical, is the neutral form of the amide ion (). Aminyl radicals are highly reactive and consequently short-lived, like most radicals; however, they form an important part of nitrogen chemistry. In sufficiently high concentration, amino radicals dimerise to form hydrazine. While as a functional group is common in nature, forming a part of many compounds (e.g. the phenethylamines), the radical cannot be isolated in its free form. Synthesis Reaction 1: Formation of amino radical from ammonia Amino radicals can be produced by reacting OH radical with ammonia in irradiated aqueous solutions. This reaction is formulated as a hydrogen abstraction reaction. :NH3 + ^\mathbfOH -> ^\mathbfNH2 + H2O The rate constant (''k1'') for this reaction was determined to be , while the parallel reaction of OH with was found to be much slower. This rate was redetermined by using two-pulse radiolysis competition met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side chain'' and '' pendant group'', are used almost interchangeably to describe those branches from the parent structure, though certain distinctions are made in polymer chemistry. In polymers, side chains extend from the backbone structure. In proteins, side chains are attached to the alpha carbon atoms of the amino acid backbone.) The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between isomers. Su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latin Language
Latin (, or , ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power of the Roman Republic it became the dominant language in the Italy (geographical region), Italian region and subsequently throughout the Roman Empire. Even after the Fall of the Western Roman Empire, fall of Western Rome, Latin remained the common language of international communication, science, scholarship and academia in Europe until well into the 18th century, when other regional vernaculars (including its own descendants, the Romance languages) supplanted it in common academic and political usage, and it eventually became a dead language in the modern linguistic definition. Latin is a fusional language, highly inflected language, with three distinct grammatical gender, genders (masculine, feminine, and neuter), six or seven ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Numerical Multiplier
The numerical multiplier (or multiplying affix) in IUPAC nomenclature indicates how many particular atoms or functional groups are attached at a particular point in a molecule. The affixes are derived from both Latin and Greek. Compound affixes The prefixes are given from the least significant decimal digit up: units, then tens, then hundreds, then thousands. For example: :548 → octa- (8) + tetraconta- (40) + pentacta- (500) = ''octatetracontapentacta-'' :9267 → hepta- (7) + hexaconta- (60) + dicta- (200) + nonalia- (9000) = ''heptahexacontadictanonalia-'' The numeral one While the use of the affix ''mono-'' is rarely necessary in organic chemistry, it is often essential in inorganic chemistry to avoid ambiguity: carbon oxide could refer to either ''carbon monoxide'' or ''carbon dioxide''. In forming compound affixes, the numeral one is represented by the term ''hen-'' except when it forms part of the number eleven (''undeca-''): hence :241 → hen- (1) + tetraconta- (40) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affix
In linguistics, an affix is a morpheme that is attached to a word stem to form a new word or word form. Affixes may be derivational, like English ''-ness'' and ''pre-'', or inflectional, like English plural ''-s'' and past tense ''-ed''. They are bound morphemes by definition; prefixes and suffixes may be separable affixes. Affixation is the linguistic process that speakers use to form different words by adding morphemes at the beginning (prefixation), the middle (infixation) or the end (suffixation) of words. Positional categories of affixes ''Prefix'' and ''suffix'' may be subsumed under the term ''adfix'', in contrast to ''infix.'' When marking text for interlinear glossing, as in the third column in the chart above, simple affixes such as prefixes and suffixes are separated from the stem with hyphens. Affixes which disrupt the stem, or which themselves are discontinuous, are often marked off with angle brackets. Reduplication is often shown with a tilde. Affixes which c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazane
Triazane is an inorganic compound with the chemical formula or . Triazane is the third simplest acyclic azane after ammonia and hydrazine. It can be synthesized from hydrazine but is unstable and cannot be isolated in the free base form, only as salt forms such as triazanium sulfate. Attempts to convert triazanium salts to the free base release only diazene and ammonia. Triazane was first synthesized as a ligand In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ... of the silver complex ion: tris(μ2-triazane-κ2''N''1,''N''3)disilver(2+). Triazane has also been synthesized in electron-irradiated ammonia ices and detected as a stable gas-phase product after sublimation.Forstel, Maksyutenko, Jones, Sun, Chen, Chang, & Kaiser. "Detection of the Elusive Triazane Molecule () in the Gas P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazane
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazines re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane Nomenclature
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like pentacontane () or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |