|
Axi-cel
Axicabtagene ciloleucel, sold under the brand name Yescarta, is a medication used for the treatment for large B-cell lymphoma that has failed conventional treatment. T cells are removed from a person with lymphoma and genetically engineered to produce a specific T-cell receptor. The resulting chimeric antigen receptor T cells (CAR-Ts) that react to the cancer are then given back to the person to populate the bone marrow. Axicabtagene treatment carries a risk for cytokine release syndrome (CRS) and neurological toxicities. Due to CD19 being a pan-B cell marker, the T-cells that are engineered to target CD19 receptors on the cancerous B cells also influence normal B cells, except some plasma cells. Side effects Because treatment with axicabtagene carries a risk of cytokine release syndrome and neurological toxicities, the FDA has mandated that hospitals be certified for its use prior to treatment of any patients. History It was developed by California-based Kite Pharma. Axicabt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Goods Administration
The Therapeutic Goods Administration (TGA) is the medicine and therapeutic regulatory agency of the Australian Government. As part of the Department of Health (Australia), Department of Health and Aged Care, the TGA regulates the quality, supply and advertising of medicines, pathology devices, medical devices, blood products and most other therapeutics. Any items that claim to have a therapeutic effect, are involved in the administration of medication, or are otherwise covered by the ''Therapeutic Goods Act 1989'', the ''Therapeutic Goods Regulations 1990'', or a ministerial order, must be approved by the TGA and registered in the Australian Register of Therapeutic Goods. Structure of the TGA and medical regulation in Australia In Australia, medical products are regulated by the TGA and, for controlled drugs such as cannabis, the Office of Drug Control (ODC). Together, the TGA and ODC form the Health Products Regulation Group within the Department of Health and Aged Care. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combinatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilead Sciences
Gilead Sciences, Inc. () is an American biopharmaceutical company headquartered in Foster City, California, that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500. Gilead was founded in 1987 under the name Oligogen by Michael L. Riordan. The original name was a reference to oligomers, small strands of DNA used to target genetic sequences. Gilead held its IPO in 1992, and successfully developed drugs like Tamiflu and Vistide that decade. In the 2000s, Gilead received approval for drugs including Viread and Hepsera, among others. It began evolving from a biotechnology company into a pharmaceutical company, acquiring several subsidiaries, though it still relied heavily on contracting to manufacture its drugs. The company continued its growth in the 2010s, but came unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Therapy
Gene therapy is a medical field which focuses on the genetic modification of cells to produce a therapeutic effect or the treatment of disease by repairing or reconstructing defective genetic material. The first attempt at modifying human DNA was performed in 1980, by Martin Cline, but the first successful nuclear gene transfer in humans, approved by the National Institutes of Health, was performed in May 1989. The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990. It is thought to be able to cure many genetic disorders or treat them over time. Between 1989 and December 2018, over 2,900 clinical trials were conducted, with more than half of them in phase I.Gene Therapy Cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer Treatments
Cancer can be treated by surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy (including immunotherapy such as monoclonal antibody therapy) and synthetic lethality, most commonly as a series of separate treatments (e.g. chemotherapy before surgery). The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient ( performance status). Cancer genome sequencing helps in determining which cancer the patient exactly has for determining the best therapy for the cancer. A number of experimental cancer treatments are also under development. Under current estimates, two in five people will have cancer at some point in their lifetime. Complete removal of the cancer without damage to the rest of the body (that is, achieving cure with near-zero adverse effects) is the ideal, if rarely achieved, goal of treatment and is often the goal in practice. Sometimes this can be accomplished ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combinatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second-line Treatment
A therapy or medical treatment (often abbreviated tx, Tx, or Tx) is the attempted remediation of a health problem, usually following a medical diagnosis. As a rule, each therapy has indications and contraindications. There are many different types of therapy. Not all therapies are effective. Many therapies can produce unwanted adverse effects. ''Medical treatment'' and ''therapy'' are generally considered synonyms. However, in the context of mental health, the term ''therapy'' may refer specifically to psychotherapy. History Before the creating of therapy as a formal procedure, people told stories to one another to inform and assist about the world. The term "healing through words" was used over 3,500 years ago in Greek and Egyptian writing. The term psychotherapy was invented in the 19th century, and psychoanalysis was founded by Sigmund Freud under a decade later. Semantic field The words ''care'', ''therapy'', ''treatment'', and ''intervention'' overlap in a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
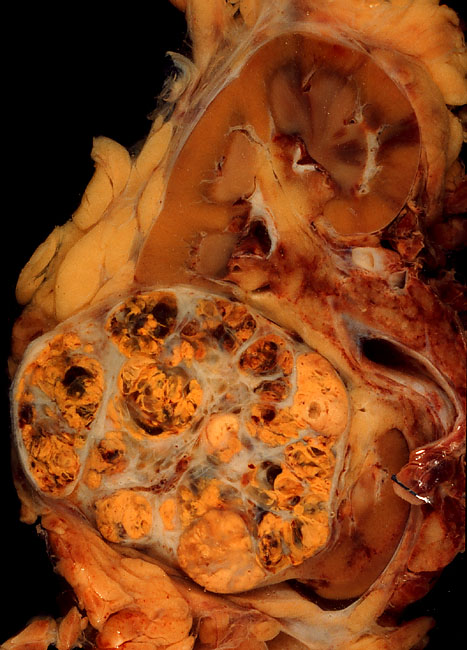
Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma (NHL), also known as non-Hodgkin's lymphoma, is a group of blood cancers that includes all types of lymphomas except Hodgkin lymphomas. Symptoms include enlarged lymph nodes, fever, night sweats, weight loss, and tiredness. Other symptoms may include bone pain, chest pain, or itchiness. Some forms are slow-growing while others are fast-growing. Lymphomas are types of cancer that develop from lymphocytes, a type of white blood cell. Risk factors include poor immune function, autoimmune diseases, ''Helicobacter pylori'' infection, hepatitis C, obesity, and Epstein–Barr virus infection. The World Health Organization classifies lymphomas into five major groups, including one for Hodgkin lymphoma. Within the four groups for NHL are over 60 specific types of lymphoma. Diagnosis is by examination of a bone marrow or lymph node biopsy. Medical imaging is done to help with cancer staging. Treatment depends on whether the lymphoma is slow- or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biologics License Application
A biologics license application (BLA) is defined by the U.S. Food and Drug Administration The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ... (FDA) as follows: The biologics license application is a request for permission to introduce, or deliver for introduction, a biologic product into interstate commerce (21 CFR 601.2). The BLA is regulated under 21 CFR 600 – 680. A BLA is submitted by any legal person or entity who is engaged in manufacture or an applicant for a license who takes responsibility for compliance with product and establishment standards. Form 356h specifies the requirements for a BLA. This includes: * Applicant information * Product/manufacturing information * Pre-clinical studies * Clinical studies * Labeling Some biological products are regulated by the Cent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug Designation
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review (FDA)
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Mediastinal B-cell Lymphoma
Primary mediastinal B-cell lymphoma, abbreviated PMBL, is a rare type of lymphoma that forms in the mediastinum (the space in between the lungs) and predominantly affects young adults. It is a subtype of diffuse large B-cell lymphoma; however, it generally has a significantly better prognosis. Diagnosis Diagnosis requires a biopsy, so that the exact type of tissue can be determined by examination under a microscope. PMBL is generally considered a sub-type of diffuse large B-cell lymphoma, although it is also closely related to nodular sclerosing Hodgkin lymphoma (NSHL). Tumors that are even more closely related to NSHL than typical for PMBL are called gray zone lymphoma. Treatment Treatment commonly begins with months of multi-drug chemotherapy regimen. Either R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) or DA-EPOCH-R (dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, rituximab) has been typical. Other, more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_I_(cropped).jpg)