|
Austenite
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902); it exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures. Allotrope of iron From alpha iron undergoes a phase transition from body-centered cubic (BCC) to the face-centered cubic (FCC) configuration of gamma iron, also called austenite. This is similarly soft and ductile but can dissolve considerably more carbon (as much as 2.03% by mass at ). This gamma form of iron is present in the most commonly used type of stainless steel for making hospital and food-service equipment. Material Austenitizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bainite
Bainite is a plate-like microstructure that forms in steels at temperatures of 125–550 °C (depending on alloy content). First described by E. S. Davenport and Edgar Bain, it is one of the products that may form when austenite (the face-centered cubic crystal structure of iron) is cooled past a temperature where it is no longer thermodynamically stable with respect to ferrite, cementite, or ferrite and cementite. Davenport and Bain originally described the microstructure as being similar in appearance to tempered martensite. A fine non-lamellar structure, bainite commonly consists of cementite and dislocation-rich ferrite. The large density of dislocations in the ferrite present in bainite, and the fine size of the bainite platelets, makes this ferrite harder than it normally would be. The temperature range for transformation of austenite to bainite (125–550 °C) is between those for pearlite and martensite. In fact, there is no fundamental lower limit to the ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
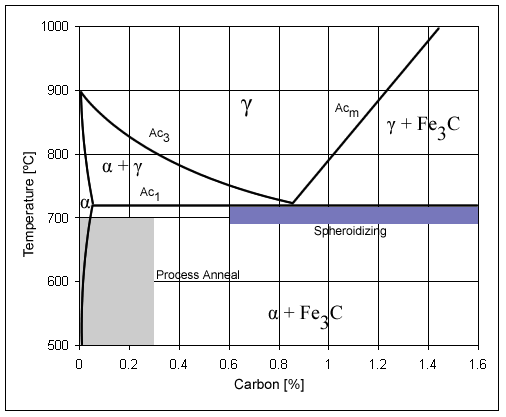
Steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant typically need an additional 11% chromium. Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, weapons, and rockets. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms (allotropic forms): body-centred cubic and face-centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties. In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quite ductile, or soft and easily formed. In steel, small amounts of carbon, other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martensite
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation. Properties Martensite is formed in carbon steels by the rapid cooling (quenching) of the austenite form of iron at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Austenite is gamma-phase iron (γ-Fe), a solid solution of iron and alloying elements. As a result of the quenching, the face-centered cubic austenite transforms to a highly strained body-centered tetragonal form called martensite that is supersaturated with carbon. The shear deformations that result produce a large number of dislocations, which is a primary strengthening mechanism of steels. The highest hardness of a pearlitic steel is 400 Brinell, whereas martensite can achieve 700 Bri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tempering (metallurgy)
Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures. Introduction Tempering is a heat treatment technique applied to ferrous alloys, such as steel or cast iron, to achieve greater toughness by decreasing the hardness of the alloy. The reduction in hardness is usually accompanied by an increase in ductility, thereby decreasing the brittleness of the metal. Tempering is usually performed after quench ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plain-carbon Steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states: * no minimum content is specified or required for chromium, cobalt, molybdenum, nickel, niobium, titanium, tungsten, vanadium, zirconium, or any other element to be added to obtain a desired alloying effect; * the specified minimum for copper does not exceed 0.40%; * or the maximum content specified for any of the following elements does not exceed the percentages noted: manganese 1.65%; silicon 0.60%; copper 0.60%. The term ''carbon steel'' may also be used in reference to steel which is not stainless steel; in this use carbon steel may include alloy steels. High carbon steel has many different uses such as milling machines, cutting tools (such as chisels) and high strength wires. These applications require a much finer microstructure, which improves the toughness. Carbon steel is a popular metal choic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, brittle material, normally classified as a ceramic in its pure form, and is a frequently found and important constituent in ferrous metallurgy. While cementite is present in most steels and cast irons, it is produced as a raw material in the iron carbide process, which belongs to the family of alternative ironmaking technologies. The name ''cementite'' originated from the theory of Floris Osmond and J. Werth, in which the structure of solidified steel consists of a kind of cellular tissue, with ferrite as the nucleus and Fe3C the envelope of the cells. The carbide therefore ''cemented'' the iron. Metallurgy In the iron–carbon system (i.e. plain-carbon steels and cast irons) it is a common constituent because ferrite can contain at most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stainless Steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corrosion resistance, resistance to corrosion results from the chromium, which forms a Passivation (chemistry), passive film that can protect the material and self-healing material, self-heal in the presence of oxygen. The alloy's properties, such as luster and resistance to corrosion, are useful in many applications. Stainless steel can be rolled into Sheet metal, sheets, plates, bars, wire, and tubing. These can be used in cookware, cutlery, surgical instruments, major appliances, vehicles, construction material in large buildings, industrial equipment (e.g., in paper mills, chemical plants, water treatment), and storage tanks and tankers for chemicals and food products. The biological cleanability of stainless steel is superior to both alumi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eutectoid
A eutectic system or eutectic mixture ( ) is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the ''eutectic temperature''. On a phase diagram, the eutectic temperature is seen as the eutectic point (see plot on the right). Non-eutectic mixture ratios would have different melting temperatures for their different constituents, since one component's lattice will melt at a lower temperature than the other's. Conversely, as a non-eutectic mixture cools down, each of its components would solidify (form a lattice) at a different temperature, until the entire mass is solid. Not all binary alloys have eutectic points, since the valence electrons of the component species are not always compatible, in any mixing ratio, to form a new type of joint crystal lattice. For example, in the silver-gold system the melt temperature (liquidus) and freeze temperature ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearlite
Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. During slow cooling of an iron-carbon alloy, pearlite forms by a eutectoid reaction as austenite cools below (the eutectoid temperature). Pearlite is a microstructure occurring in many common grades of steels. The eutectoid composition of austenite is approximately 0.8% carbon; steel with less carbon content ( hypoeutectoid steel) will contain a corresponding proportion of relatively pure ferrite crystallites that do not participate in the eutectoid reaction and cannot transform into pearlite. Likewise steels with higher carbon content ( hypereutectoid steels) will form cementite before reaching the eutectoid point. The proportion of ferrite and cementite forming above the eutectoid point can be calculated from the iron/iron—carbide equilibrium phase diagram using the lever rule. Steels with p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Iron
At atmospheric pressure, three allotropic forms of iron exist, depending on temperature: alpha iron (α-Fe), gamma iron (γ-Fe), and delta iron (δ-Fe). At very high pressure, a fourth form exists, called epsilon iron (ε-Fe). Some controversial experimental evidence suggests the existence of a fifth high-pressure form that is stable at very high pressures and temperatures. The phases of iron at atmospheric pressure are important because of the differences in solubility of carbon, forming different types of steel. The high-pressure phases of iron are important as models for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist essentially of a crystalline iron-nickel alloy with ε structure. The outer core surrounding the solid inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter elements. Standard pressure allotropes Alpha iron (α-Fe) Below 912 °C (1,674 °F), iron has a bod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Iron
At atmospheric pressure, three allotropic forms of iron exist, depending on temperature: alpha iron (α-Fe), gamma iron (γ-Fe), and delta iron (δ-Fe). At very high pressure, a fourth form exists, called epsilon iron (ε-Fe). Some controversial experimental evidence suggests the existence of a fifth high-pressure form that is stable at very high pressures and temperatures. The phases of iron at atmospheric pressure are important because of the differences in solubility of carbon, forming different types of steel. The high-pressure phases of iron are important as models for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist essentially of a crystalline iron-nickel alloy with ε structure. The outer core surrounding the solid inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter elements. Standard pressure allotropes Alpha iron (α-Fe) Below 912 °C (1,674 °F), iron has a bod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martensitic Transformation
A diffusionless transformation is a phase change that occurs without the long-range diffusion of atoms but rather by some form of cooperative, homogenous movement of many atoms that results in a change in the crystal structure. These movements are small, usually less than the interatomic distances, and the neighbors of an atom remain close. The systematic movement of large numbers of atoms led to some to refer to these as ''military'' transformations in contrast to ''civilian'' diffusion-based phase changes, initially by Frederick Charles Frank and John Wyrill Christian. The most commonly encountered transformation of this type is the martensitic transformation which, while probably the most studied, is only one subset of non-diffusional transformations. The martensitic transformation in steel represents the most economically significant example of this category of phase transformations, but an increasing number of alternatives, such as shape memory alloys, are becoming more impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |