|
Astragalus Membranaceus
''Astragalus propinquus'' (syn. ''Astragalus membranaceus'', commonly known as Mongolian milkvetch in English; 'Хунчир' in Mongolian; ''huáng qí'' (), ''běi qí'' () or ''huáng huā huáng qí'' (), in Mongolia, is a flowering plant in the family Fabaceae. It is one of the 50 fundamental herbs used in traditional Mongolian medicine. It is a perennial plant and it is not listed as being threatened. Herbalism ''A. propinquus'' is used in traditional Chinese medicine (TCM). ''A. propinquus'' is a component in Lectranal, a food supplement used in treatment of seasonal allergic rhinitis, though there is limited evidence of its effectiveness. Chemistry Chemical constituents of the roots (Radix Astragali) include polysaccharides and triterpenoids (such as astragalosides), as well as isoflavones (including kumatakenin, calycosin, and formononetin) and their glycosides and malonates. An extract of ''A. propinquus'' called TA-65 may activate telomerase, extending th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schischkin
Boris Konstantinovich Schischkin (born 1886 in Kukarka; died 21 March 1963 in Leningrad) was a Russian botanist and from 1943 corresponding member of the Academy of Sciences of the USSR. His name was russian: Борис Константинович Шишкин, with his surname sometimes transliterated as Shishkin. Life and Works In 1911 Schischkin graduated from the Faculty of Medicine of Tomsk State University and from 1913 to 1915 he taught there. Between 1915 and 1918 he worked as a military medical officer. From 1918 to 1925 he headed the botanical section of the Caucasian Museum in Tbilisi. From 1925 to 1930 he was professor at the Tomsk State University, where he held a chair of morphology and plant systematics. From 1930 he worked in the Komarov Botanical Institute of the Academy of Sciences of the USSR (from 1938 to 1949 as director) and from 1945 to 1958 also as professor at Leningrad University. From 1946 to 1963 he was vice-president of the Botanical Society of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calycosin
Calycosin is an ''O''-methylated isoflavone. It can be isolated from ''Astragalus membranaceus'' Bge. var. ''mongholicus'' and ''Trifolium pratense'' L. (red clover). Biosynthesis Isoflavone 3′-hydroxylase uses formononetin Formononetin is an O-methylated isoflavone. Natural occurrences Formononetin is found in a number of plants and herbs such as red clover. Along with other phytoestrogens, it predominantly occurs in leguminous plants and Fabaceae, particularly i ..., NADPH, H+ and O2 to produce calycosin, NADP+ and H2O. References O-methylated isoflavones {{phenol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astragalus
''Astragalus'' is a large genus of over 3,000 species of herbs and small shrubs, belonging to the legume family Fabaceae and the subfamily Faboideae. It is the largest genus of plants in terms of described species. The genus is native to temperate regions of the Northern Hemisphere. Common names include milkvetch (most species), locoweed (in North America, some species) and goat's-thorn ( ''A. gummifer'', ''A. tragacantha''). Some pale-flowered vetches (''Vicia'' spp.) are similar in appearance, but they are more vine-like than ''Astragalus''. Description Most species in the genus have pinnately compound leaves. There are annual and perennial species. The flowers are formed in clusters in a raceme, each flower typical of the legume family, with three types of petals: banner, wings, and keel. The calyx is tubular or bell-shaped. Ecology ''Astragalus'' species are used as food plants by the larvae of some Lepidoptera species including many case-bearing moths of the genus ''Col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tremella Fuciformis
''Tremella fuciformis'' is a species of fungus; it produces white, frond-like, gelatinous basidiocarps (fruiting bodies). It is widespread, especially in the tropics, where it can be found on the dead branches of broadleaf trees. This fungus is commercially cultivated and is one of the most popular fungi in the cuisine and medicine of China. ''Tremella fuciformis'' is commonly known as snow fungus, snow ear, silver ear fungus, white jelly mushroom, and white cloud ears. ''Tremella fuciformis'' is a parasitic yeast, and grows as a slimy, mucus-like film until it encounters its preferred hosts, various species of '' Annulohypoxylon'' (or possibly ''Hypoxylon'') fungi, whereupon it then invades, triggering the aggressive mycelial growth required to form the fruiting bodies. Taxonomy and naming ''Tremella fuciformis'' was first described in 1856 by English mycologist Miles Joseph Berkeley, based on collections made in Brazil by the botanist and explorer Richard Spruce. In 1939, Ja ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacopoeia
A pharmacopoeia, pharmacopeia, or pharmacopoea (from the obsolete typography ''pharmacopœia'', meaning "drug-making"), in its modern technical sense, is a book containing directions for the identification of compound medicines, and published by the authority of a government or a medical or pharmaceutical society. Descriptions of preparations are called monographs. In a broader sense it is a reference work for pharmaceutical drug specifications. Etymology The term derives from grc, φαρμακοποιία ''pharmakopoiia'' "making of (healing) medicine, drug-making", a compound of φάρμακον ''pharmakon'' "healing medicine, drug, poison", the verb ποιεῖν ''poiein'' "to make" and the abstract noun suffix -ία ''-ia''. In early modern editions of Latin texts, the Greek diphthong οι (''oi'') is latinized to its Latin equivalent ''oe'' which is in turn written with the ligature ''œ'', giving the spelling ''pharmacopœia''; in modern UK English, ''œ'' is wri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dietary Supplement
A dietary supplement is a manufactured product intended to supplement one's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources or that are synthetic in order to increase the quantity of their consumption. The class of nutrient compounds includes vitamins, minerals, fiber, fatty acids, and amino acids. Dietary supplements can also contain substances that have not been confirmed as being essential to life, but are marketed as having a beneficial biological effect, such as plant pigments or polyphenols. Animals can also be a source of supplement ingredients, such as collagen from chickens or fish for example. These are also sold individually and in combination, and may be combined with nutrient ingredients. The European Commission has also established harmonized rules to help insure that food supplements are safe and appropriately labeled. Creating an industry estimated to have a 2020 value of $ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium – from Greek ( 'Moon') – was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitro Compound
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid. Synthesis Preparation of aromatic nitro compounds Aromatic nitro compounds are typically synthesized by nitration. Nitration is achieved using a mixture of nitric acid and sulfuric acid, which produce the nitronium ion (), which is the electrophile: + The nitration product produced on the la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , Medicinal plant, plants, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indolizine
Indolizine is an heterocyclic compound with the formula C8H7N). It is an uncommon isomer of indole with the nitrogen located at a ring fusion position. The saturated analogs are indolizidine, which are found in a variety of alkaloids such as swainsonine Swainsonine is an indolizidine alkaloid. It is a potent inhibitor of Golgi alpha-mannosidase II, an immunomodulator, and a potential chemotherapy drug. As a toxin in locoweed (likely its primary toxin) it also is a significant cause of economi .... References External links Chemical synthesis of indolizines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloastragenol
Cycloastragenol is a triterpenoid isolated from various legume species in the genus ''Astragalus'' that is purported to have telomerase activation activity. A preliminary ''in vitro'' study on human CD4 and CD8 T cells found that cycloastragenol may moderately increase telomerase activity and inhibit the onset of cellular senescence. History Cycloastragenol was patented by Geron Corporation and sold to Telomerase Activation Sciences in early 2013 who are developing it as a product named TA-65. Bill Andrews of Sierra Sciences has done testing on the anti- aging aspect of TA-65;, as well as Maria Blasco in the journal ''Aging Cell'', finding no increase in murine median or mean lifespan but some physiological anti-aging effects without augmenting cancer incidence. TA sciences was served with a consent order by the Federal Trade Commission for deceptive advertising implying that TA-65 can reverse aging and repair DNA damage. Potential pharmacology Its mode of action is purpo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
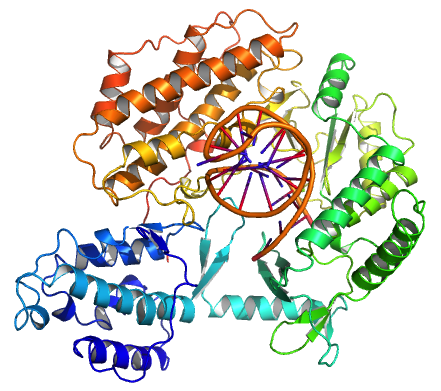
Telomerase
Telomerase, also called terminal transferase, is a ribonucleoprotein that adds a species-dependent telomere repeat sequence to the 3' end of telomeres. A telomere is a region of repetitive sequences at each end of the chromosomes of most eukaryotes. Telomeres protect the end of the chromosome from DNA damage or from fusion with neighbouring chromosomes. The fruit fly ''Drosophila melanogaster'' lacks telomerase, but instead uses retrotransposons to maintain telomeres. Telomerase is a reverse transcriptase enzyme that carries its own RNA molecule (e.g., with the sequence 3′- CCC AA UCCC-5′ in ''Trypanosoma brucei'') which is used as a template when it elongates telomeres. Telomerase is active in gametes and most cancer cells, but is normally absent from, or at very low levels in, most somatic cells. History The existence of a compensatory mechanism for telomere shortening was first found by Soviet biologist Alexey Olovnikov in 1973, who also suggested the telomere hypothe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_silver_ear_mushroom.jpg)