|
Apigenin Biosynthesis
Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool. Sources in nature Apigenin is found in many fruits and vegetables, but parsley, celery, celeriac, and chamomile tea are the most common sources. Apigenin is particularly abundant in the flowers of chamomile plants, constituting 68% of total flavonoids. Dried parsley can contain about 45 mg apigenin/gram of the herb, and dried chamomile flower about 3-5 mg/gram. The apigenin content of fresh parsley is reportedly 215.5 mg/100 grams, which is much higher than the next highest food source, green celery hearts providing 19.1 mg/100 grams. Biosynthesis Apigenin is biosynthetically derived from the general phenylpropanoid pathway and the flavone synthesis pathway. The phenylpropanoid pathway starts from the aromatic amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merck Index
''The Merck Index'' is an encyclopedia of chemical substance, chemicals, pharmaceutical drug, drugs and biomolecule, biologicals with over 10,000 monographs, monograph on single substances or groups of related chemical compound, compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Merck Group, Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also include ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans-cinnamate 4-monooxygenase
In enzymology, a trans-cinnamate 4-monooxygenase () is an enzyme that catalyzes the chemical reaction :trans-cinnamate + NADPH + H+ + O2 \rightleftharpoons 4-hydroxycinnamate + NADP+ + H2O The 4 substrates of this enzyme are trans-cinnamate, NADPH, H+, and O2, whereas its 3 products are 4-hydroxycinnamate, NADP+, and H2O. This enzyme participates in phenylalanine metabolism and phenylpropanoid biosynthesis. It employs one cofactor, heme. This enzyme belongs to the family of oxidoreductases, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with NADH or NADPH as one donor, and incorporation of one atom o oxygen into the other donor. Nomenclature The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhoifolin
Rhoifolin is a chemical compound. It is first isolated from plant ''Rhus succedanea''. The term "Rhoi" derived from generic name of plant Rhus. It is a flavone, a type of flavonoid isolated from '' Boehmeria nivea'', China grass or ramie (leaf), from ''Citrus limon'', Canton lemon (leaf), from ''Citrus x aurantium'', the bigarade or bitter orange (plant), from ''Citrus x paradisi'', the grapefruit (leaf), from '' Ononis campestris'', the cammock (shoot) and from ''Sabal serratula'', the serenoa ''Serenoa repens'', commonly known as saw palmetto, is the sole species currently classified in the genus ''Serenoa''. It is a small palm, growing to a maximum height around . It is endemic to the subtropical and tropical Southeastern United S ... or sabal fruit (plant). References Flavone glycosides {{Aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isovitexin
Isovitexin (or homovitexin, saponaretin) is a flavone. the apigenin-6-''C''-glucoside. In this case, the prefix 'iso' does not imply an isoflavonoid (the position of the B-ring on the C-ring), but the position of the glucoside on the flavone. Natural occurrence It can be found in the passion flower, Cannabis, oat and the açaí palm."Pharmacological studies of Passiflora sp. and their bioactive compounds" Metabolism * Isovitexin beta-glucosyltransferase Glycosides Saponarin is the isovitexin-7-O-glucoside. See also * Vitexin, the 8-C-glucoside of apigenin * Isoorientin, the 3'-OH derivative References {{flavone Flavone glucosides C-glycoside natural phenols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitexin
Vitexin is an apigenin flavone glucoside, a chemical compound found in the passion flower, ''Vitex agnus-castus'' (chaste tree or chasteberry), in the ''Phyllostachys nigra'' bamboo leaves, in the pearl millet (Pennisetum millet), and in Hawthorn. Metabolism Goitrogenicity of millet flavones : Vitexin inhibits thyroid peroxidase thus contributing to goiter. * Vitexin beta-glucosyltransferase * Vitexin 2"-O-rhamnoside 7-O-methyltransferase See also * Isovitexin (or homovitexin, saponaretin) is the apigenin-6-''C''-glucoside. * Orientin Orientin is a flavone, a chemical flavonoid-like compound. It is the 8-C glucoside of luteolin. Natural occurrences Orientin is found in ''Adonis vernalis'', in '' Anadenanthera colubrina'' and ''Anadenanthera peregrina'', and in the '' Phyllost ..., the 3'-OH derivative References External links Vitexin on RDchemicals.com {{flavone Flavone glucosides C-glycoside natural phenols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dandelion Coffee
Dandelion 'coffee' (also dandelion tea) is a tisane made from the root of the dandelion plant. The roasted dandelion root pieces and the beverage have some resemblance to coffee in appearance and taste, and it is thus commonly considered a coffee substitute. Dandelion root is used for both medicinal and culinary purposes and is thought to be a detoxifying herb. History The usage of the dandelion plant dates back to the ancient Egyptians, Greeks and Romans. Additionally, for over a thousand years, Chinese traditional medicine has been known to incorporate the plant. Susanna Moodie explained how to prepare dandelion 'coffee' in her memoir of living in Canada, ''Roughing it in the Bush'' (1852), where she mentions that she had heard of it from an article published in the 1830s in ''New York Albion'' by a certain Dr. Harrison. Dandelion 'coffee' was later mentioned in a '' Harpers New Monthly Magazine'' story in 1886. In 1919, dandelion root was noted as a source of cheap 'coffee' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apigetrin
Apigetrin is a chemical compound that can be found in dandelion coffee and in ''Teucrium gnaphalodes ''Teucrium gnaphalodes'' is a plant species in the genus ''Teucrium''. It is endemic to the Iberian Peninsula and grows at altitudes between 200 and 1500 m. It flowers from March to July. The flavones diosmin, cirsimaritin, salvigenin, cirsili ...''. References External links Apigetrin on chemlink.com Flavone glucosides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apiin
Apiin is a natural flavonoid, a diglycoside of the flavone apigenin found in the winter-hardy plants parsley and celery, and in banana leaf. The glycoside moiety at carbon-7 of apigenin, ''O''-β-D-apiofuranosyl(→)2-β-D-glucosyl, is carried by several other flavones in parsley plant and seed. The sugar apiose possibly play a role in winter hardiness of celery, duckweed and parsley.page 136 "Advances in Carbohydrate Chemistry and Biochemistry", Volume 31, See also *Apiose Apiose is a branched-chain sugar found as residues in galacturonans-type pectins; that occurs in parsley and many other plants. Apiose is a component of cell wall polysaccharides. Apiose 1-reductase uses D-apiitol and NAD+ to produce api ... References Flavone glycosides {{Aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a '' C-glycoside'') glycosidic bond. According to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcone Isomerase
In enzymology, a chalcone isomerase () is an enzyme that catalyzes the chemical reaction :a chalcone \rightleftharpoons a flavanone Hence, this enzyme has one substrate, a chalcone, and one product, a flavanone. This enzyme belongs to the family of isomerases, specifically the class of intramolecular lyases. The systematic name of this enzyme class is flavanone lyase (decyclizing). This enzyme is also called chalcone-flavanone isomerase. This enzyme participates in flavonoid biosynthesis. The ''Petunia hybrida'' (Petunia) genome contains two genes coding for very similar enzymes, ChiA and ChiB, but only the first seems to encode a functional chalcone isomerase. Structural studies As of late 2007, 7 structures have been solved for this class of enzymes, with PDB accession codes , , , , , , and . Chalcone isomerase has a core 2-layer alpha/beta structure A structure is an arrangement and organization of interrelated elements in a material object or system, or the object ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid. Functions It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis. Fatty acid biosynthesis Malonyl-CoA provides 2-carbon units to fatty acids and commits them to fatty acid chain synthesis. Malonyl-CoA is formed by carboxylating acetyl-CoA using the enzyme acetyl-CoA carboxylase. One molecule of acetyl-CoA joins with a molecule of bicarbonate,Nelson D, Cox M (2008) ''Lehninger principles of biochemistry''. 5th Ed: p. 806 requiring energy rendered from ATP. Malonyl-CoA is utilised in fatty acid biosynthesis by the enzyme malonyl coenzyme A:acyl carrier protein transacylase (MCAT). MCAT serves to transfer malonate from malonyl-CoA to the terminal thiol of ''holo''-acyl carrier protein (ACP). Polyketide biosynthesis MCAT is also involved in bacterial polyketide biosynthesis. The enzyme MCAT together with an acyl carrier protein (ACP), and a polyketide synthase (PKS) and chain-length f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
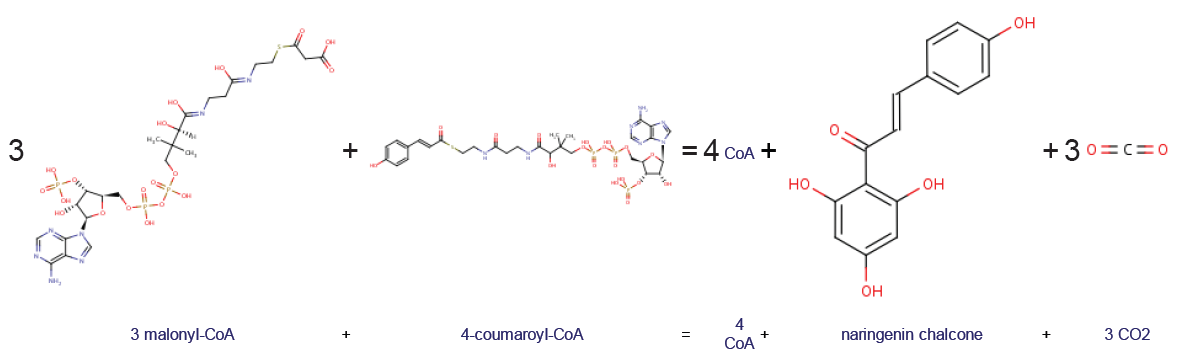
Chalcone Synthase
Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compounds found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid biosynthesis. The enzyme catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone. Function CHS catalysis serves as the initial step for flavonoid biosynthesis. Flavonoids are important plant secondary metabolites that serve various functions in higher plants. These include pigmentation, UV protection, fertility, antifungal defense and the recruitment of nitrogen-fixing bacteria. CHS is believed to act as a central hub for the enzymes involved in the flavonoid pathway. Studies have shown that these enzyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |