|
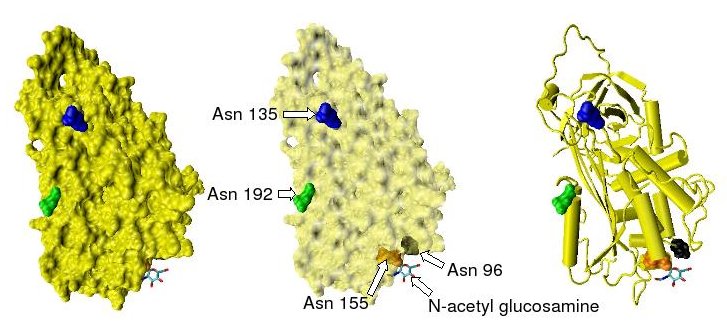
Antithrombin
Antithrombin (AT) is a small glycoprotein that inactivates several enzymes of the coagulation system. It is a 432-amino-acid protein produced by the liver. It contains three disulfide bonds and a total of four possible glycosylation sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manyfold by the anticoagulant drug heparin, which enhances the binding of antithrombin to factor IIa (prothrombin) and factor Xa. Nomenclature Antithrombin is also termed antithrombin III (AT III). The designations antithrombin I through to antithrombin IV originate in early studies carried out in the 1950s by Seegers, Johnson and Fell. Antithrombin I (AT I) refers to the absorption of thrombin onto fibrin after thrombin has activated fibrinogen. Antithrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Protease Inhibitor
Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases (serine protease inhibitors). They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site. Protease inhibition by serpins controls an array of biological processes, including coagulation and inflammation, and consequently these proteins are the target of medical research. Their unique conformational change also makes them of interest to the structural biology and protein folding research communities. The conformatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coagulation
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin. Coagulation begins almost instantly after an injury to the endothelium lining a blood vessel. Exposure of blood to the subendothelial space initiates two processes: changes in platelets, and the exposure of subendothelial tissue factor to plasma factor VII, which ultimately leads to cross-linked fibrin formation. Platelets immediately form a plug at the site of injury; this is called ''primary hemostasis. Secondary hemostasis'' occurs simultaneously: additional coagulation (clotting) factors beyond factor VII ( listed below) respond in a cascade to form fibrin strands, which strengthen the platelet plug. Disorder ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticoagulant
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide. Anticoagulants are closely related to antiplatelet drugs and thrombolytic drugs by manipulating the various pathways of blood coagulation. Specifically, antiplatelet drugs inhibit plate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Coagulation
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin. Coagulation begins almost instantly after an injury to the endothelium lining a blood vessel. Exposure of blood to the subendothelial space initiates two processes: changes in platelets, and the exposure of subendothelial tissue factor to plasma factor VII, which ultimately leads to cross-linked fibrin formation. Platelets immediately form a plug at the site of injury; this is called ''primary hemostasis. Secondary hemostasis'' occurs simultaneously: additional coagulation (clotting) factors beyond factor VII ( listed below) respond in a cascade to form fibrin strands, which strengthen the platelet plug. Dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heparin Cofactor II
Heparin cofactor II (HCII), a protein encoded by the SERPIND1 gene, is a coagulation factor that inhibits IIa, and is a cofactor for heparin and dermatan sulfate ("minor antithrombin"). The product encoded by this gene is a serine protease inhibitor which rapidly inhibits thrombin in the presence of dermatan sulfate or heparin. The gene contains five exons and four introns. This protein shares homology with antithrombin III and other members of the alpha-1 antitrypsin superfamily. Mutations in this gene are associated with heparin cofactor II deficiency. Heparin cofactor II deficiency can lead to increased thrombin generation and a hypercoagulable state. A purification experiment of heparin cofactor II was performed in 1981, in which it was discovered that the purified version of the protein consists of a single polypeptide chain. Further experimentation demonstrated that whether β-Mercaptoethanol is present does not affect HCII's activity in gel electrophoresis. β-Mercaptoetha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoprotein
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recombinant DNA
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome. Recombinant DNA is the general name for a piece of DNA that has been created by combining at least two fragments from two different sources. Recombinant DNA is possible because DNA molecules from all organisms share the same chemical structure, and differ only in the nucleotide sequence within that identical overall structure. Recombinant DNA molecules are sometimes called chimeric DNA, because they can be made of material from two different species, like the mythical chimera. R-DNA technology uses palindromic sequences and leads to the production of sticky and blunt ends. The DNA sequences used in the construction of recombinant DNA molecules can originate from any species. For example, plant DNA may be joined to ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, cryo-electron microscopy, and submitted by biologists and biochemists from around the world, are freely accessible on the Internet via the websites of its member organisations (PDBe, PDBj, RCSB, and BMRB). The PDB is overseen by an organization called the Worldwide Protein Data Bank, wwPDB. The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other databases use protein structures deposited in the PDB. For example, SCOP and CATH classify protein structures, while PDBsum provides a graphic overview of PDB entries using information from other sources, such as Gene ontology. History Two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baculovirus
''Baculoviridae'' is a family of viruses. Arthropods, among the most studied being Lepidoptera, Hymenoptera and Diptera, serve as natural hosts. Currently, 85 species are placed in this family, assigned to four genera. Baculoviruses are known to infect insects, with over 600 host species having been described. Immature (larval) forms of lepidopteran species (moths and butterflies) are the most common hosts, but these viruses have also been found infecting sawflies, and mosquitoes. Although baculoviruses are capable of entering mammalian cells in culture, they are not known to be capable of replication in mammalian or other vertebrate animal cells. Starting in the 1940s, they were used and studied widely as biopesticides in crop fields. Baculoviruses contain a circular, double-stranded DNA (dsDNA) genome ranging from 80 to 180 kbp. Historical influence The earliest records of baculoviruses can be found in the literature from as early as the 16th century in reports of "wilting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |