|
Antisense Oligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small bits of nucleic acids can be manufactured as single-stranded molecules with any user-specified sequence, and so are vital for artificial gene synthesis, polymerase chain reaction (PCR), DNA sequencing, molecular cloning and as molecular probes. In nature, oligonucleotides are usually found as small RNA molecules that function in the regulation of gene expression (e.g. microRNA), or are degradation intermediates derived from the breakdown of larger nucleic acid molecules. Oligonucleotides are characterized by the sequence of nucleotide residues that make up the entire molecule. The length of the oligonucleotide is usually denoted by "-mer" (from Greek ''meros'', "part"). For example, an oligonucleotide of six nucleotides (nt) is a hexamer, while one of 25 nt woul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular mass.'' The name is composed of Greek elements '' oligo-'', "a few" and '' -mer'', "parts". An adjective form is ''oligomeric''. The oligomer concept is contrasted to that of a polymer, which is usually understood to have a large number of units, possibly thousands or millions. However, there is no sharp distinction between these two concepts. One proposed criterion is whether the molecule's properties vary significantly with the removal of one or a few of the units. An oligomer with a specific number of units is referred to by the Greek prefix denoting that number, wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hybridization Probe
In molecular biology, a hybridization probe (HP) is a fragment of DNA or RNA of usually 15–10000 nucleotide long which can be radioactively or fluorescently labeled. HP can be used to detect the presence of nucleotide sequences in analyzed RNA or DNA that are complementary to the sequence in the probe. The labeled probe is first denatured (by heating or under alkaline conditions such as exposure to sodium hydroxide) into single stranded DNA (ssDNA) and then hybridized to the target ssDNA ( Southern blotting) or RNA (northern blotting) immobilized on a membrane or in situ. To detect hybridization of the probe to its target sequence, the probe is tagged (or "labeled") with a molecular marker of either radioactive or (more recently) fluorescent molecules. Commonly used markers are 32P (a radioactive isotope of phosphorus incorporated into the phosphodiester bond in the probe DNA), digoxigenin, a non-radioactive, antibody-based marker, biotin or fluorescein. DNA sequences o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleic Acid Structure
Nucleic acid structure refers to the structure of nucleic acids such as DNA and RNA. Chemically speaking, DNA and RNA are very similar. Nucleic acid structure is often divided into four different levels: primary, secondary, tertiary, and quaternary. Primary structure Primary structure consists of a linear sequence of nucleotides that are linked together by phosphodiester bond. It is this linear sequence of nucleotides that make up the primary structure of DNA or RNA. Nucleotides consist of 3 components: # Nitrogenous base ## Adenine ## Guanine ## Cytosine ## Thymine (present in DNA only) ## Uracil (present in RNA only) # 5-carbon sugar which is called deoxyribose (found in DNA) and ribose (found in RNA). # One or more phosphate groups. The nitrogen bases adenine and guanine are purine in structure and form a glycosidic bond between their 9 nitrogen and the 1' -OH group of the deoxyribose. Cytosine, thymine, and uracil are pyrimidines, hence the glycosidic bonds form betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organothiophosphate
Organothiophosphates or organophosphorothioates are a subclass of organophosphorus compounds. Many are used as pesticides, some have medical applications, and some are used as oil additives. They generally have the chemical formula (RO)3PS, RO)2P(S)Osup>−, R(RO)2PS, etc. Oligonucleotide phosphorothioates (OPS) are modified oligonucleotides where one of the oxygen atoms in the phosphate moiety is replaced by sulfur. These compounds are the basis of antisense therapy, e.g., the drugs fomivirsen (Vitravene), Oblimersen, Alicaforsen, and Mipomersen (Kynamro). Further examples of these include: * Diazinon * Fenitrothion * Fenthion * Thiotepa Variants with P=S double bonds were developed as insecticides because of their reduced mammalian toxicity. The phosphorothioate P=S bond is converted to the toxic P=O bond in the target insect. Similar oxidative conversion in mammals is slower, conferring lower toxicity in mammals. Structure and chemical synthesis Generally these compounds fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-performance Liquid Chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column. HPLC has been used for manufacturing (''e.g.'', during the production process of pharmaceutical and biological products), legal (''e.g.'', detecting performance enhancement drugs in urine), research (''e.g.'', separating the components of a complex biological sample, or of similar synthetic chemicals from each other), and medical (''e.g.'', detecting vitamin D levels in blood serum) purposes. Chrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building-blocks of DNA and RNA. List of nucleosides and corresponding nucleobases The reason for 2 symbols, shorter and longer, is that the shorter ones are better for contexts where explicit disambiguation is superfluous (because context disambiguates) and the longer ones are for contexts where explicit disambiguation is judged to be needed or wise. For example, when discussing long nucleobase sequences in genomes, the CATG symbol system is much preferable to the Cyt-Ade-Thy-Gua symbol system (see '' N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
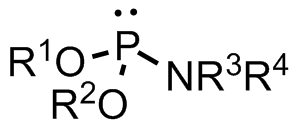
Phosphoramidite
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety. Applications Nucleoside phosphoramidites Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs. As ligands Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis. A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondary amines. This type of ligand was first used in 1996 in an asymmetric copper-catalysed addition of dialkylzincs to enones. See also * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antisense Therapy
Antisense therapy is a form of treatment that uses antisense oligonucleotides (ASOs) to target messenger RNA (mRNA). ASOs are capable of altering mRNA expression through a variety of mechanisms, including ribonuclease H mediated decay of the pre-mRNA, direct steric blockage, and exon content modulation through splicing site binding on pre-mRNA. Several ASOs have been approved in the United States, the European Union, and elsewhere. Nomenclature The common stem for antisense oligonucleotides drugs is -rsen. The substem -virsen designates antiviral antisense oligonucleotides. Pharmacokinetics and pharmacodynamics Half-life and stability ASO-based drugs employ highly modified, single-stranded chains of synthetic nucleic acids that achieve wide tissue distribution with very long half-lives. For instance, many ASO-based drugs contain phosphorothioate substitutions and 2' sugar modifications to inhibit nuclease degradation enabling vehicle-free delivery to cells. ''In vivo'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent In Situ Hybridization
Fluorescence ''in situ'' hybridization (FISH) is a molecular cytogenetic technique that uses fluorescent probes that bind to only particular parts of a nucleic acid sequence with a high degree of sequence complementarity. It was developed by biomedical researchers in the early 1980s to detect and localize the presence or absence of specific DNA sequences on chromosomes. Fluorescence microscopy can be used to find out where the fluorescent probe is bound to the chromosomes. FISH is often used for finding specific features in DNA for use in genetic counseling, medicine, and species identification. FISH can also be used to detect and localize specific RNA targets (mRNA, lncRNA and miRNA) in cells, circulating tumor cells, and tissue samples. In this context, it can help define the spatial-temporal patterns of gene expression within cells and tissues. Probes – RNA and DNA In biology, a probe is a single strand of DNA or RNA that is complementary to a nucleotide sequence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |