|
Antimicrobial Peptide Resistance And Lipid A Acylation Protein Family
Antimicrobial peptide resistance and lipid A acylation protein PagP is a family of several bacterial antimicrobial peptide resistance and lipid A acylation (PagP) proteins. The bacterial outer membrane enzyme PagP transfers a palmitate chain from a phospholipid to lipid A. In a number of pathogenic Gram-negative bacteria, PagP confers resistance to certain cationic antimicrobial peptides produced during the host innate immune response The innate, or nonspecific, immune system is one of the two main immunity strategies (the other being the adaptive immune system) in vertebrates. The innate immune system is an older evolutionary defense strategy, relatively speaking, and is the .... References Protein domains Protein families Outer membrane proteins {{membrane-protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Family
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with Family (biology), family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar protein structure, three-dimensional structures, functions, and significant Sequence homology, sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
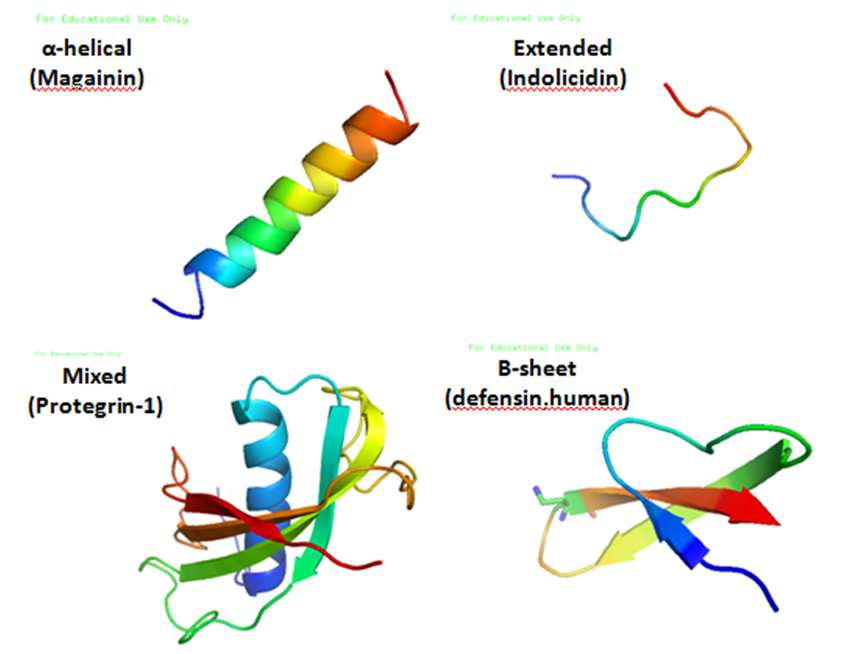
Antimicrobial Peptide
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid A
Lipid A is a lipid component of an endotoxin held responsible for the toxicity of gram-negative bacteria. It is the innermost of the three regions of the lipopolysaccharide (LPS), also called endotoxin molecule, and its hydrophobic nature allows it to anchor the LPS to the outer membrane. While its toxic effects can be damaging, the sensing of lipid A by the immune system may also be critical for the onset of immune responses to gram-negative infection, and for the subsequent successful fight against the infection. Chemical composition Lipid A consists of two glucosamine (carbohydrate/sugar) units, in an β(1→6) linkage, with attached acyl chains ("fatty acids"), and normally containing one phosphate group on each carbohydrate. The optimal immune activating lipid A structure is believed to contain 6 acyl chains. Four acyl chains attached directly to the glucosamine sugars are beta hydroxy acyl chains usually between 10 and 16 carbons in length. Two additional acyl chains are oft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent. Because they form a strong electrophile when treated with some metal catalysts, acyl halides are commonly used as acylating agents. For example, Friedel–Crafts acylation uses acetyl chloride (ethanoyl chloride or ) as the agent and aluminum chloride () as a catalyst to add an ethanoyl ( acetyl) group to benzene: The mechanism of this reaction is electrophilic aromatic substitution. Acyl halides and acid anhydrides of carboxylic acids are also commonly used acylating agents. In some cases, active esters exhibit comparable reactivity. All react with amines to form amides and with alcohols to form esters by nucleophilic acyl substitution. Acylation can be used to prevent rearrangement reactions that would normally occur in alkylation. To do this an acylation reaction is performed, then the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-negative Bacteria
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane. Gram-negative bacteria are found in virtually all environments on Earth that support life. The gram-negative bacteria include the model organism ''Escherichia coli'', as well as many pathogenic bacteria, such as ''Pseudomonas aeruginosa'', '' Chlamydia trachomatis'', and ''Yersinia pestis''. They are a significant medical challenge as their outer membrane protects them from many antibiotics (including penicillin), detergents that would normally damage the inner cell membrane, and lysozyme, an antimicrobial enzyme produced by animals that forms part of the innate immune system. Additionally, the outer leaflet of this membrane comprises a complex lipopol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Innate Immune Response
The innate, or nonspecific, immune system is one of the two main immunity strategies (the other being the adaptive immune system) in vertebrates. The innate immune system is an older evolutionary defense strategy, relatively speaking, and is the dominant immune system response found in plants, fungi, insects, and primitive multicellular organisms (see Beyond vertebrates).. The major functions of the innate immune system are to: * recruit immune cells to infection sites by producing chemical factors, including chemical mediators called cytokines * activate the complement cascade to identify bacteria, activate cells, and promote clearance of antibody complexes or dead cells * identify and remove foreign substances present in organs, tissues, blood and lymph, by specialized white blood cells * activate the adaptive immune system through antigen presentation * act as a physical and chemical barrier to infectious agents; via physical measures such as skin and chemical measures such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein familie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |