|
Angle Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic. In any case, the general form of the chemical formula for cycloalkanes is C''n''H2(' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adolf Von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer (; 31 October 1835 – 20 August 1917) was a German chemist who synthesised indigo and developed a nomenclature for cyclic compounds (that was subsequently extended and adopted as part of the IUPAC organic nomenclature). He was ennobled in the Kingdom of Bavaria in 1885 and was the 1905 recipient of the Nobel Prize in Chemistry.''Adolf von Baeyer: Winner of the Nobel Prize for Chemistry 1905 '' Armin de Meijere Angewandte Chemie International Edition Volume 44, Issue 48, Pages 7836 – 7840 2005''Abstract/ref> Family and education Baeyer was born in Berlin as the son of the noted geodesist and captain of the Royal Prussian Army Johann Jacob Baeyer and his wife Eugenie Baeyer née Hitzig (1807–1843). Both his parents were Lutherans at the time of his birth and he was raised in the Lutheran religion. His mother was the daughter of Julius Eduard Hitzig and a member of the originally Jewish Itzig family, and had converted to Christianity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Angles
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can give molecula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Newman Projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman projection visualizes the conformation of a chemical bond from front to back, with the front atom represented by the intersection of three lines (a dot) and the back atom as a circle. The front atom is called ''proximal'', while the back atom is called ''distal''. This type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms. This projection is named after American chemist Melvin Spencer Newman, who introduced it in 1952 as a partial replacement for Fischer projections, which are unable to represent conformations and thus conformers properly.Newman, MS. ''Record. Chem. Progr. (Kresge-Hooker Sci. Lib.) 1952,'' 13'', 111'' This diagram style is an alternative to a sawhorse projection, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrophotometry
Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths. Overview Spectrophotometry is a tool that hinges on the quantitative analysis of molecules depending on how much light is absorbed by colored compounds. Important features of spectrophotometers are spectral bandwidth (the range of colors it can transmit through the test sample), the percentage of sample-transmission, the logarithmic range of sample-absorption, and sometimes a percentage of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans-Cyclooctene
''trans''-Cyclooctene is a cyclic hydrocarbon with the formula ��(CH2)6CH=CH– where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor. Cyclooctene is notable as the smallest cycloalkene that is readily isolated as its ''trans''-isomer. The ''cis''-isomer is much more stable; the ring-strain energies being 16.7 and 7.4 kcal/mol, respectively.Ron Walker, Rosemary M. Conrad, and Robert H. Grubbs (2009): "The living ROMP of ''trans''-cyclooctene". ''Macromolecules'', volume 42, issue 3, pages 599–605. A planar arrangement of the ring carbons would be too strained, and therefore the stable conformations of the ''trans'' form have a bent (non-planar) ring. Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring. A "half-chair" conformation, with about 6 kcal/mol higher energy, has carbons 2,3,5,6, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
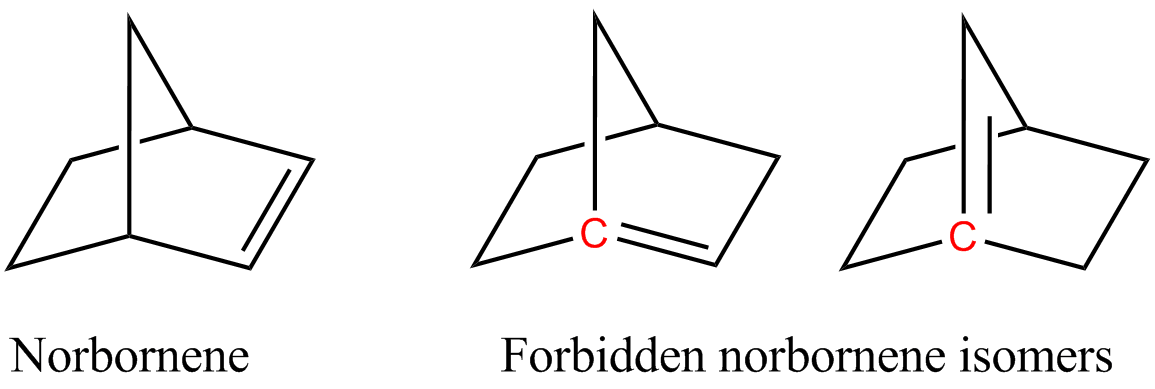
Norbornene Isomers Bredt Rule
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity. Production Norbornene is made by a Diels–Alder reaction of cyclopentadiene and ethylene. Many substituted norbornenes can be prepared similarly. Related bicyclic compounds are norbornadiene, which has the same carbon skeleton but with two double bonds, and norbornane which is prepared by hydrogenation of norbornene. Reactions Norbornene undergoes an acid-catalyzed hydration reaction to form norborneol. This reaction was of great interest in the elucidation of the non-classical carbonion controversy. Norbornene is used in the Catellani reaction and in norbornene-mediated ''meta''-C−H activation. Certain substituted norbornenes undergo unusual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bredt's Rule
Bredt's rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. It primarily relates to bridgeheads with carbon-carbon and carbon-nitrogen double bonds. For example, two of the following isomers of norbornene violate Bredt's rule, which makes them too unstable to prepare: In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red. Bredt's rule is a consequence of the fact that having a double bond on a bridgehead, carbons from which three bonds radiate and which the rings share a single covalent bond, would be equivalent to having a trans double bond on a ring, which is not stable for small rings (fewer than eight atoms) due to a combination of ring strain, and angle strain (nonplanar alkene). The p orbitals of the bridgehead ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded to its three neighbors. Buckminsterfullerene is a black solid that dissolves in hydrocarbon solvents to produce a violet solution. The compound was discovered in 1985 and has received intense study, although few real world applications have been found. Occurrence Buckminsterfullerene is the most common naturally occurring fullerene. Small quantities of it can be found in soot. It also exists in space. Neutral C60 has been observed in planetary nebulae and several types of star. The ionised form, C60+, has been identified in the interstellar medium, where it is the cause of several absorption features known as diffuse interstellar bands in the near-infrared. History Theoretical predictions of buckyball molecules appeared in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as , where the '<' denotes the two bonds. This can equally well be represented as . This stands in contrast to a situation where the carbon atom is bound to the rest of the molecule by a double bond, which is preferably called a , represented . Formerly the methylene name was used for both isomers. The name ““ can be used for the single-bonded isomer, to emphatically exclude methylidene. The distinction is often important, because the double bond is chemically di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |