|
Amyl Formate
Amyl may refer to: * Amylum or starch, a carbohydrate ** Amylopectin, a polymer of glucose found in plants; one of two components of starch ** Amylose, a helical polymer made of α-D-glucose units; one of two components of starch * Pentyl, a five-carbon alkyl functional group, also known by the common non-systematic name amyl * Amyl nitrite, used to treat heart diseases and cyanide poisoning (known as Amyl when used as a recreational drug) *Dinitrogen tetroxide Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium ..., an oxidizer used in rocket fuel See also * Amylamine, a solvent and raw material * Amylase, an enzyme that catalyses the hydrolysis of starch into sugars * Amyloid, fibrous protein aggregates, originally named in the mistaken belief that they contained starch * Amyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylum
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc). Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules: the linear and helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the energy reserve of animals, is a more highly branched version of amylopectin. In industry, starch is often converted into sugars, for example by malting. These sugars may be fermented to produce ethanol in the manufacture of beer, whisky and biofuel. In addition, sugars produced from processed starch are used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
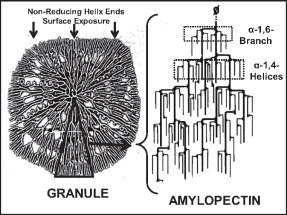
Amylopectin
Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose. Plants store starch within specialized organelles called amyloplasts. To generate energy, the plant hydrolyzes the starch, releasing the glucose subunits. Humans and other animals that eat plant foods also use amylase, an enzyme that assists in breaking down amylopectin, to initiate the hydrolyzation of starch. Starch is made of about 70–80% amylopectin by weight, though it varies depending on the source. For example, it ranges from lower percent content in long-grain rice, amylomaize, and russet potatoes to 100% in glutinous rice, waxy potato starch, and waxy corn. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units. Its inner chains are formed of 20–24 glucose subunits. Dissolved amylopectin starch has a lower tendency of retrogradation (a partial recrystallization a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
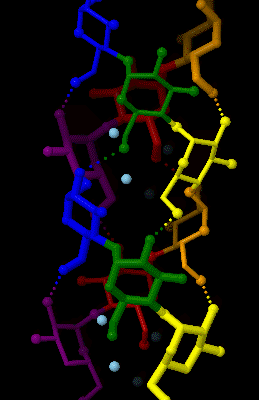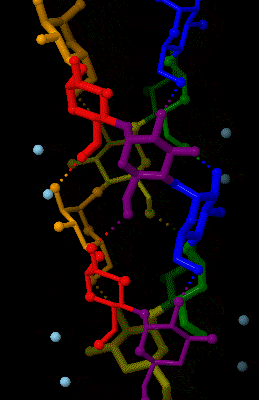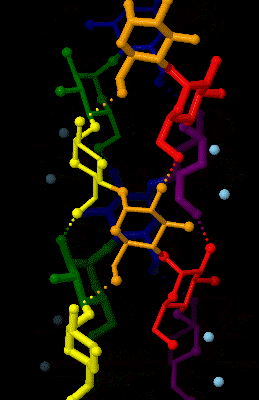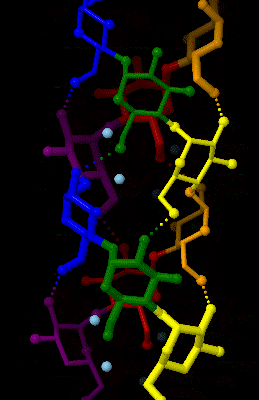
Amylose
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–30%. Because of its tightly packed helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix (A or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentyl
Pentyl is a five-carbon alkyl group or substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane. In older literature, the common non-systematic name amyl was often used for the pentyl group. Conversely, the name pentyl was used for several five-carbon branched alkyl groups, distinguished by various prefixes. The nomenclature has now reversed, with "amyl" being more often used to refer to the terminally branched group also called isopentyl, as in amobarbital. A cyclopentyl group is a ring with the formula -C5H9. The name is also used for the pentyl radical, a pentyl group as an isolated molecule. This free radical is only observed in extreme conditions. Its formula is often written "•" or "• " to indicate that it has one unsatisfied valence bond. Older "pentyl" groups The following names are still used sometimes: Pentyl radical The free radical pentyl was studied by J. Pacansky and A. Gutierrez in 1983. The radical was obtained by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyl Nitrite
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It is also referred to as ''banapple gas''. It was first documented in 1844 and came into medical use in 1867. Uses * Amyl nitrite is employed medically to treat heart diseases as well as angina. * Amyl nitrite is sometimes used as an antidote for cyanide poisoning. It can act as an oxidant, to induce the formation of methemoglobin. Methemoglobin in turn can sequester cyanide as cyanomethemoglobin. * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Tetroxide
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol. Dinitrogen tetroxide is a powerful oxidizer that is hypergolic (spontaneously reacts) upon contact with various forms of hydrazine, which has made the pair a common bipropellant for rockets. Structure and properties Dinitrogen tetroxide could be regarded as two nitro groups (-NO2) bonded together. It forms an equilibrium mixture with nitrogen dioxide. The molecule is planar with an N-N bond distance of 1.78Å and N-O distances of 1.19Å. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1.45Å. This exceptionally weak σ bond (amounting to overlapping of the ''sp''2 hybrid orbitals of the two NO2 units) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylamine
1-Aminopentane is an organic compound with the formula CH3(CH2)4NH2. It is used as a solvent, as a raw material in the manufacture of a variety of other compounds, including dyes, emulsifiers, and pharmaceutical products, and as a flavoring agent.Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke, "Amines, Aliphatic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Pentylamine exhibits reactions typical of other simple alkyl amines, i.e. protonation, alkylation, acylation, condensation with carbonyls. Like other simple aliphatic amines, pentylamine is a weak base A weak base is a base that, upon dissolution in water, does not dissociate completely, so that the resulting aqueous solution contains only a small proportion of hydroxide ions and the concerned basic radical, and a large proportion of undissociat ...: the pKa of H3(CH2)4NH3sup>+ is 10.21. See also * 3-Aminopentane References {{DEFAULTSORT:Aminopentane, 1- Alkylamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylase
An amylase () is an enzyme that catalyses the hydrolysis of starch (Latin ') into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase (alpha amylase) to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase. Specific amylase proteins are designated by different Greek letters. All amylases are glycoside hydrolases and act on α-1,4-glycosidic bonds. Classification α-Amylase The α-amylases () ( CAS 9014-71-5) (alternative names: 1,4-α-D-glucan glucanohydrolase; glycogenase) are calcium metalloenzymes. By acting at random loca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid
Amyloids are aggregates of proteins characterised by a Fibril, fibrillar morphology of 7–13 Nanometer, nm in diameter, a beta sheet (β-sheet) Secondary structure of proteins, secondary structure (known as cross-β) and ability to be Staining, stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal Protein structure, structure and physiology, physiological functions (Protein misfolding, misfolding) and form fibrous deposits in amyloid plaques around cells which can disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are Genetic disorder, familial. Others are only Genetic disorder, fam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloplast
Amyloplasts are a type of plastid, double-enveloped organelles in plant cells that are involved in various biological pathways. Amyloplasts are specifically a type of leucoplast, a subcategory for colorless, non-pigment-containing plastids. Amyloplasts are found in roots and storage tissues, and they store and synthesize starch for the plant through the polymerization of glucose. Starch synthesis relies on the transportation of carbon from the cytosol, the mechanism by which is currently under debate. Starch synthesis and storage also takes place in chloroplasts, a type of pigmented plastid involved in photosynthesis. Amyloplasts and chloroplasts are closely related, and amyloplasts can turn into chloroplasts; this is for instance observed when potato tubers are exposed to light and turn green. Role in gravity sensing Amyloplasts are thought to play a vital role in gravitropism. Statoliths, a specialized starch-accumulating amyloplast, are denser than cytoplasm, and are able to se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyl Acetate
Amyl acetate (pentyl acetate) is an organic compound and an ester with the chemical formula CH3COO H2sub>4CH3 and the molecular weight 130.19g/mol. It is colorless and has a scent similar to bananas and apples. The compound is the condensation product of acetic acid and 1-pentanol. However, esters formed from other pentanol isomers (amyl alcohols), or mixtures of pentanols, are often referred to as amyl acetate. The symptoms of exposure to amyl acetate in humans are dermatitis, central nervous system depression, narcosis and irritation to the eyes and nose. Uses It is used as a flavoring agent, as a paint and lacquer solvent, and in the preparation of penicillin. It is an inactive ingredient in liquid bandages. It is used as a fuel in the Hefner lamp. See also *Isoamyl acetate, also known as banana oil. *Ester In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyl Alcohol
An amyl alcohol is any of eight alcohols with the formula C5H12O. A mixture of pentyl, amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol. Amyl alcohol is used as a solvent and in esterification, by which is produced amyl acetate and other important products. The name ''amyl alcohol'' without further specification applies to the normal (straight-chain) form, 1-Pentanol, 1-pentanol. These are the 8 alcohols that are structural isomers with molecular formula C5H12O: : Three of these alcohols, 2-methyl-1-butanol, 2-pentanol, and 3-methyl-2-butanol (methyl isopropyl carbinol), are therefore optical isomerism, optically active. The most important amyl alcohol is isoamyl alcohol, the chief one generated by fermentation in the production of alcoholic beverages and a constituent of fusel oil. The other amyl alcohols may be obtained synthetically. References {{DEFAULTSORT:Amyl Alcohol Alkanols GABAA receptor positive allosteric modulators ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |