|
Ammoxidation
In organic chemistry, ammoxidation is a process for the production of nitriles () using ammonia () and oxygen (). It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually: :CH3CH=CH2 + 3/2 O2 + NH3 -> N#CCH=CH2 + 3 H2O Scope Ammoxidation of alkenes exploits the weak C-H bonds that are located in the allylic position of unsaturated hydrocarbons. Benzylic C-H bonds are also susceptible to ammoxidation, reflecting the weakness of their C-H bonds. Benzonitrile is produced from toluene, and phthalonitriles are produced from xylenes. The reaction represents a partial oxidation. Many byproducts are generated, but the feedstocks are often simple, which compensates for these losses. Additionally, some byproducts are useful or recyclable. For the production of acrylonitrile, byproducts include hydrogen cyanide, acrole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons. Inorganic compounds containing the group are not called nitriles, but cyanides instead. Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic. Structure and basic properties The N−C−C geometry is linear in nitriles, reflecting the sp hybridization of the triply bonded carbon. The C−N distance is short at 1.16 Å, consistent with a triple bond. Nitril ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niacin (substance)
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds. Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms of pellagra include skin and mouth lesions, anemia, headaches, and tiredness. Many countries mandate its addition to wheat flour or other food grains, thereby reducing the risk of pellagra. The amide derivative nicotinamide (niacinamide) is a component of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP+). Although niacin and nicotinamide are identical in their vitamin activity, nicotinam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
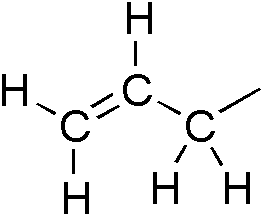
Acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular structure, it consists of a vinyl group () linked to a nitrile (). It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses. Acrylonitrile was first synthesized by the French chemist Charles Moureu (1863–1929) in 1893. Occurrence Acrylonitrile is not naturally formed on Earth. It has been detected at the sub-ppm level at industrial sites. It persists in the air for up to a week. It decomposes by reacting with oxygen and hydroxyl radical to form formyl cyanide and formaldehyde. Acrylonitrile is harmful to aquatic life. Acrylonitrile has been detected in the atmosphere of Titan, a moon of Saturn. Computer simulations suggest that on Titan conditions exi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-cyanopyridine
Nicotinonitrile or 3-cyanopyridine is an organic compound with the formula NCC5H4N. The molecule consists of a pyridine ring with a nitrile group attached to the 3-position. A colorless solid, it is produced by ammoxidation of 3-methylpyridine: :H3CC5H4N + NH3 + 1.5 O2 → NCC5H4N + 3 H2O Nicotinonitrile is a precursor to the vitamin niacin. Nitrilase-catalyzed hydrolysis of 3-cyanopyridine by means of immobilized '' Rhodococcus rhodochrous'' J1 strains leads in quantitative yield to nicotinamide (vitamin B3). The enzyme allows for a more selective synthesis as further hydrolysis of the amide to nicotinic acid Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variet ... is avoided. Oxidation of 3-cyanopyridine with hydrogen peroxide gives 3-cyanopyridine N-oxide, which hydrolyze ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzonitrile
Benzonitrile is the chemical compound with the formula , abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine. Production It is prepared by ammoxidation of toluene, that is its reaction with ammonia and oxygen (or air) at . : + 3/2 + → + In the laboratory it can be prepared by the dehydration of benzamide or by the Rosenmund–von Braun reaction using cuprous cyanide or NaCN/ DMSO and bromobenzene. : Applications Laboratory uses Benzonitrile is a useful solvent and a versatile precursor to many derivatives. It reacts with amines to afford N-substituted benzamides after hydrolysis. It is a precursor to diphenylketimine (b.p. 151 °C, 8 mm Hg) via reaction with phenylmagnesium bromide followed by methanolysis. Benzonitrile forms coordination complexes with transition metals that are both soluble in organic solvents and conveniently labile. One ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value. The mixture is referred to as both xylene and, more precisely, xylenes. Mixed xylenes refers to a mixture of the xylenes plus ethylbenzene. The four compounds have identical empirical formulas . Typically the four compounds are produced together by various catalytic reforming and pyrolysis methods. Occurrence and production Xylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. They also occur in crude oil in concentrations of about 0.5–1%, depending on the source. Small quantities occur in gasoline and aircraft f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phthalonitrile
Phthalonitrile is an organic compound with the formula C6H4(CN)2, which is an off-white crystal solid at room temperature. It is a derivative of benzene, containing two adjacent nitrile groups. The compound has low solubility in water but is soluble in common organic solvents. The compound is used as a precursor to phthalocyanine and other pigments, fluorescent brighteners, and photographic sensitizers. Synthesis Phthalonitrile is produced industrially in a single-stage continuous process, by the ammoxidation of ''o''-xylene at 480 °C. The reaction is catalyzed by vanadium oxide-antimony-oxide in a fluidized bed reactor.Lorz, Peter M. "Phthalic Acid and Derivatives" in Ulmanns Encyclopedia of Industrial Chemistry. Wiley-VCH: Weinheim, 2002. . : Phthalonitrile was first described in 1896 by Johannes Pinnow. It was noted as a byproduct of the synthesis of ortho-dicyanodiazoamidobenzene via the reaction of ortho-amidobenzonitrile hydrochloride, sodium nitrite, and hydroc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Chlorobenzonitrile
4-Chlorobenzonitrile is an organic compound with the formula ClC6H4CN. It is a white solid. The compound, one of three isomers of chlorobenzonitrile, is produced industrially by ammoxidation of 4-chlorotoluene. The compound is of commercial interest as a precursor to pigment A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic comp ...s. References {{DEFAULTSORT:Chlorobenzonitrile, 4- Benzonitriles Chlorobenzenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Chlorobenzonitrile
2-Chlorobenzonitrile is an organic compound with the formula ClC6H4CN. It is a white solid. The compound, one of three isomers of chlorobenzonitrile, is produced industrially by ammoxidation of 2-chlorotoluene Chlorotoluene is a group of three isomeric chemical compounds. They (''ortho''-chlorotoluene, ''meta''-chlorotoluene, and ''para''-chlorotoluene) consist of a disubstituted benzene ring with one chlorine atom and one methyl group. Properties The .... The compound is of commercial interest as a precursor to 2-amino-5-nitrobenzonitrile, a precursor to dyes. References {{DEFAULTSORT:Chlorobenzonitrile, 2- Benzonitriles Chlorobenzenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. Structure and general properties Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide. Hydrogen cyanide is weakly acidic with a p''K''a of 9.2. It partially ionizes in water solution to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called ''hydrocyanic acid''. The salts of the cyan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_space_filling_model.jpg)