|
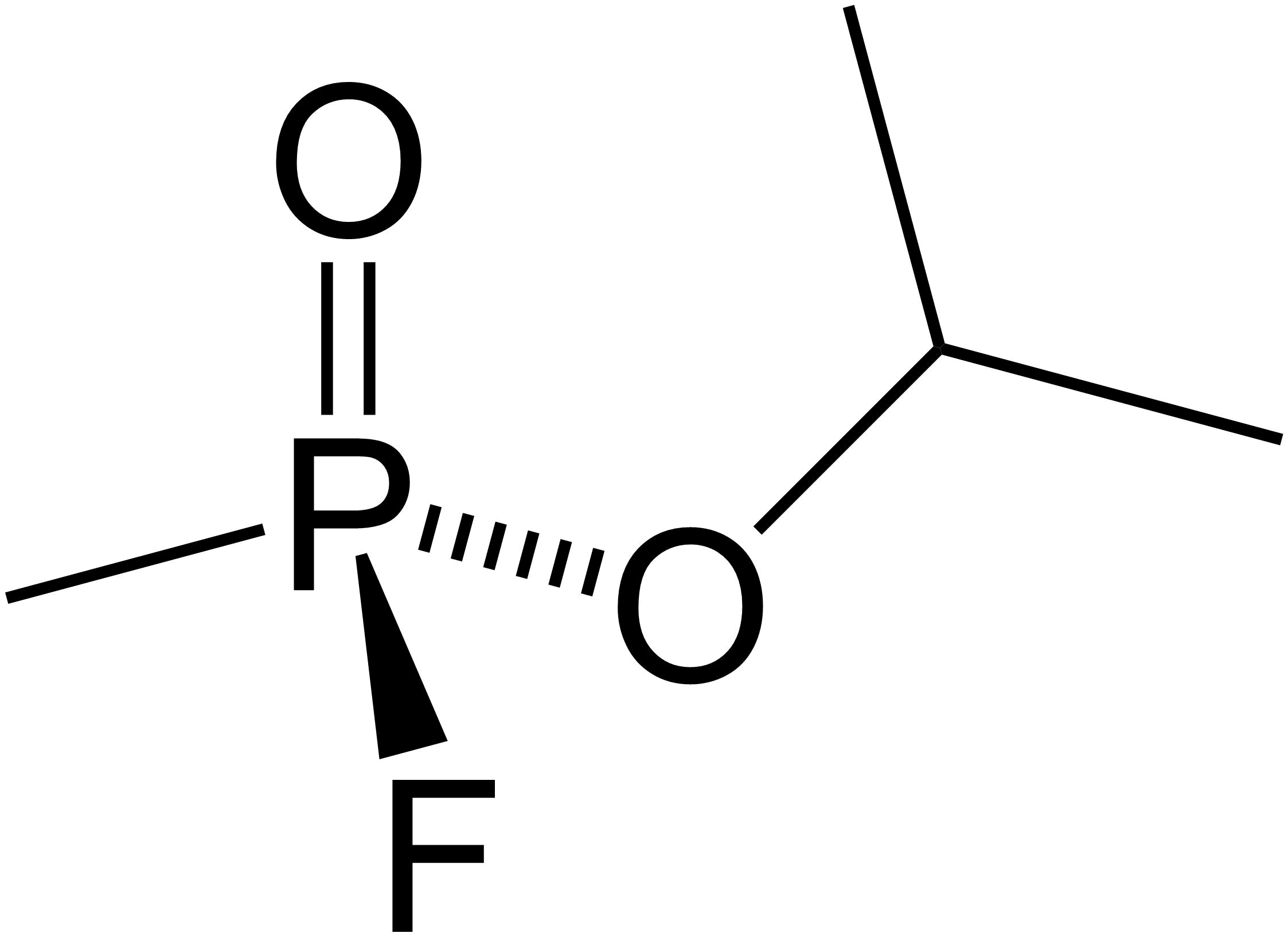
Aminal
In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: . (As is customary in organic chemistry, R can represent hydrogen or an alkyl group). A common aminal is bis(dimethylamino)methane, a colorless liquid that is prepared by the reaction of dimethylamine and formaldehyde: : 2 (CH3)2NH + CH2O -> CH3)2NCH2 + H2O Aminals are encountered in, for instance, the Fischer indole synthesis. Several examples exist in nature. Physostigmin.svg, Physostigmine,a highly toxic cholinesterase inhibitor found in the Calabar bean. Hodgkinsine.png, Hodgkinsine, an alkaloid with antiviral, antibacterial and antifungal effects 5,10-methylenetetrahydrofolic acid.svg, 5,10-Methylenetetrahydrofolate, an intermediate in one-carbon metabolism Hexahydro-1,3,5-triazine (), an intermediate in the condensation of formaldehyde and ammonia, tends to degrade to hexamethylene tetraamine. Cyclic aminal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemiaminal
In organic chemistry, a hemiaminal (also carbinolamine) is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: . R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution. Hemiaminals can be viewed as a blend of aminals and geminal diol. They are a special case of amino alcohols. Classification according to amine precursor Addition of ammonia The adducts formed by the addition of ammonia to aldehydes have long been studied. Compounds containing both a primary amino group and a hydroxyl group bonded to the same carbon atom are rare. They are invoked but rarely observed as intermediates in the reaction of ammonia and aldehydes and ketones. One example of this rare functionality is the adduct of ammonia and hexafluoroacetone, . The C-substituted derivatives are obtained by reaction of aldehydes and ammonia: :3 RCHO + 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazolidine
Imidazolidine is a heterocyclic compound (CH2)2(NH)2CH2. The parent imidazolidine is lightly studied, but related compounds substituted on one or both nitrogen centers are more common. Generally, they are colorless, polar, basic compounds. Imidazolidines are cyclic 5-membered examples of the general class of aminals. Preparation Imidazolidines are traditionally prepared by condensation reaction of 1,2-diamines and aldehydes. Most commonly, one or both nitrogen center is substituted with an alkyl or benzyl (Bn) group:Ferm, R. J.; Riebsomer, J. L. From "The chemistry of the 2-imidazolines and imidazolidines" Chemical Reviews, 1954, 54, 593-613. :(CH2NBn)2 + PhCHO → (CH2NBn)2C(H)Ph + H2O The first unsubstituted imidazolidine synthesis was reported in 1952. Reactions Unsubstituted imidazolidines are often labile. The rings are susceptible to hydrolysis back to the diamine and the aldehyde. Formally, removal of the two hydrogens at carbon 2 (between the two nitrogens) w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 1 carbon: methylenediamine (diaminomethane) of theoretical interest only * 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. File:Ethylene_diamine.png, Ethylenediamine * 3 carbons: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbons: putrescine (butane-1,4-diamine) * 5 carbons: cadaverine (pentane-1,5-diamine) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamethylene Tetraamine
Hexamethylenetetramine, also known as methenamine, hexamine, or urotropin, is a heterocyclic organic compound with the formula (CH2)6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other organic compounds, including plastics, pharmaceuticals, and rubber additives. It sublimes in vacuum at 280 °C. Synthesis, structure, reactivity Hexamethylenetetramine was discovered by Aleksandr Butlerov in 1859. In this article, Butlerov discovered formaldehyde, which he called "dioxymethylen" (methylene dioxide) age 247because his empirical formula for it was incorrect (C4H4O4). On pages 249–250, he describes treating formaldehyde with ammonia gas, creating hexamine. It is prepared industrially by combining formaldehyde and ammonia: : The reaction can be conducted in gas phase and in solution. The molecule has a tetrahedral cage-like structure, similar t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5,10-Methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer R5,10-methylene-THF. As an intermediate in one-carbon metabolism, 5,10-CH2-THF interconverts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR) It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase. Selected functions Formaldehyde detoxification Methylenetetrahydrofolate is an intermediate in the detoxification of formaldehyde. Pyrimidine biosynthesis It is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate ( dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP). The coenzyme is necessary for the biosynthesis of thymidine and is the C1-donor in the reactions catalyzed by TS and thymidyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial
An antimicrobial is an agent that kills microorganisms or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. Agents that kill microbes are microbicides, while those that merely inhibit their growth are called bacteriostatic agents. The use of antimicrobial medicines to treat infection is known as antimicrobial chemotherapy, while the use of antimicrobial medicines to prevent infection is known as antimicrobial prophylaxis. The main classes of antimicrobial agents are disinfectants (non-selective agents, such as bleach), which kill a wide range of microbes on non-living surfaces to prevent the spread of illness, antiseptics (which are applied to living tissue and help reduce infection during surgery), and antibiotics (which destroy microorganisms within the body). The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , Medicinal plant, plants, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calabar Bean
''Physostigma venenosum'', the Calabar bean or ordeal bean, is a leguminous plant, Endemic to tropical Africa, with a seed poisonous to humans. It derives the first part of its scientific name from a curious beak-like appendage at the end of the stigma, in the centre of the flower; this appendage, though solid, was supposed to be hollow (hence the name from , a bladder, and stigma). Growth The plant is a large, herbaceous, climbing perennial, with the stem woody at the base, up to in diameter; it has a habit like the scarlet runner, and attains a height of about . The flowers, appearing in axillary peduncles, are large, about long, grouped in pendulous, fascicled racemes pale-pink or purplish, and heavily veined. The seed pods, which contain two or three seeds or beans, are in length; and the beans are about the size of an ordinary horse bean but less flattened, with a deep chocolate-brown color. Toxicology Calabar bean contains physostigmine, a reversible cholinesterase i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholinesterase Inhibitor
Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and butyrylcholinesterase inhibitors (BChEIs). ChEIs may be used as drugs for Alzheimer's and myasthenia gravis, and also as chemical weapons and insecticides. Side effects when used as drugs may include loss of appetite, nausea, vomiting, loose stools, vivid dreams at night, dehydration, rash, bradycardia, peptic ulcer disease, seizures, weight loss, rhinorrhea, salivation, muscle cramps, and fasciculations. ChEIs are indirect-acting parasympathomimetic drugs. ChEls are widely used as chemical weapons. Since November 2019 the group of ACheIs known as ''Novic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |