|
Alkene Cis Effect
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis–trans Isomerism
''Cis''–''trans'' isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "''cis''" and "''trans''" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, ''cis'' indicates that the functional groups (substituents) are on the same side of some plane, while ''trans'' conveys that they are on opposing (transverse) sides. ''Cis''–''trans'' isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. ''Cis-trans'' notation does not always correspond to ''E''–''Z'' isomerism, which is an ''absolute'' stereochemical description. In general, ''cis''–''trans'' stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Butene
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting ''cis''/''trans''-isomerism (also known as (''E''/''Z'')-isomerism); that is, it exists as two geometric isomers ''cis''-but-2-ene ((''Z'')-but-2-ene) and ''trans-''but-2-ene ((''E'')-but-2-ene). It is a petrochemical, produced by the catalytic cracking of crude oil or the dimerization of ethylene. Its main uses are in the production of gasoline (petrol) and butadiene,. although some but-2-ene is also used to produce the solvent butanone via hydration to 2-butanol followed by oxidation. The two isomers are extremely difficult to separate by distillation because of the proximity of their boiling points (~4 °C for ''cis'' and ~1 °C for ''trans'' ). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Butene
1-Butene (or 1-Butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas that is easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin. It is one of the isomers of butene (butylene). It is a precursor to diverse products. Reactions Polymerization of 1-butene give polybutene, which is used to make piping for domestic plumbing. Its main application is as a comonomer in the production of certain kinds of polyethylene, such as linear low-density polyethylene (LLDPE). It has also been used as a precursor to polypropylene resins, butylene oxide, and butanone. Manufacturing 1-Butene is produced by separation from crude C4 refinery streams and by ethylene dimerization. The former affords a mixture of 1-and 2-butenes, while the latter affords only the terminal alkene. It is distilled to give a very high purity product. An estimated 12 billion kilograms were produced in 2011. See also *Butene * Dimer (chemistry) *Octene O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor. Production Steam cracking The dominant technology for producing propylene is steam cracking. The same technology is applied to ethane to ethylene. These two conversions are the #2 and #1 processes in the chemical industry, as judged by their scale. In this process, propane undergoes dehydrogenation. The by-product is hydrogen: :CH3CH2CH3 → CH3CH=CH2 + H2 The yield of propene is about 85 m%. By-products are usually used as fuel for the propane dehydrogenation reaction. Steam cracking is one of the most energy-intensive industrial processes. The feedstock is naphtha or propane, especially in the Middle East, where there is an abundance of propane from oil/gas operations. Propene can be separated by fractional di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains are classified as small ( cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium ( cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic. In any case, the general form of the chemical formula for cycloalkanes is C''n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which the atoms and bonding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
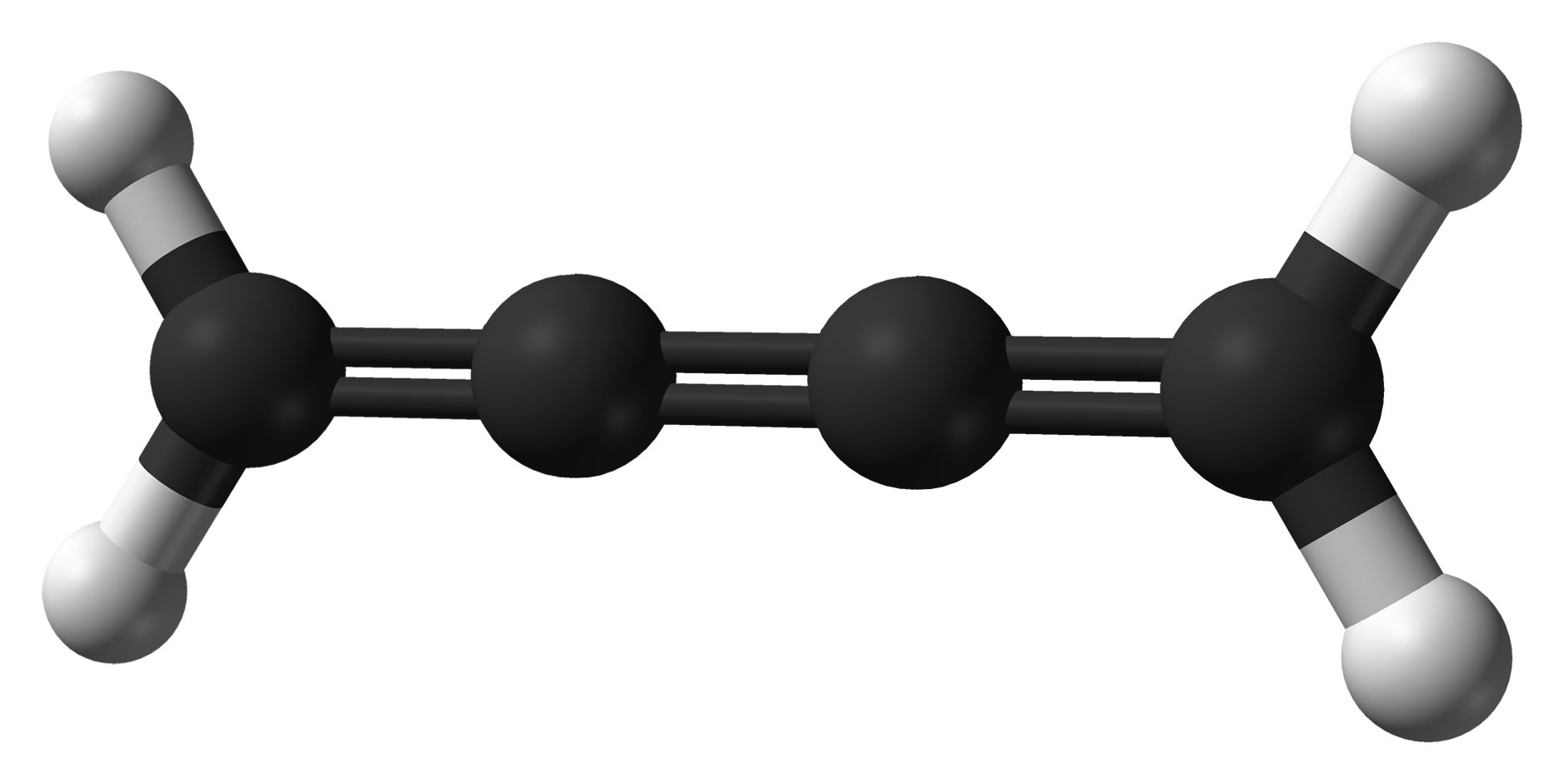
Cumulene
In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular clouds and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reported synthesis of a butatriene is that of tetraphenylbutatriene in 1921. The most common synthetic method for butatriene synthesis is based on reductive coupling of a geminal dihalo vinylidene. Tetraphenylbutatriene was reported synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propadiene
Propadiene () or allene () is the organic compound with the formula . It is the simplest allene, i.e. a compound with two adjacent carbon double bonds. As a constituent of MAPP gas, it has been used as a fuel for specialized welding. Production and equilibrium with methylacetylene Allene exists in equilibrium with methylacetylene (propyne) and the mixture is sometimes called MAPD for ''m''ethyl''a''cetylene-''p''ropa''d''iene: :H3CC#CH <<=> H2C=C=CH2 for which at 270 °C or 0.1 at 5 °C. MAPD is produced as a side product, often an undesirable one, of dehydrogenation of propane to produce , an important [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allenes
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called ''allene''. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with bonding. History For many years, allenes were viewed as curiosities but thought to be synthetically useless and difficult to prepare and to work with.The Chemistry of the Allenes (vol. 1−3); Landor, S. R., Ed.; cademic Press: London, 1982. Reportedly, the first synthesis of an allene, glutinic acid, was performed in an attempt to prove the non-existence of this class of compounds. The situation began to change in the 1950s, and more than 300 papers on allenes have been published in 2012 alone. These compounds are not just interesting intermediates but syntheti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Liv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)