|
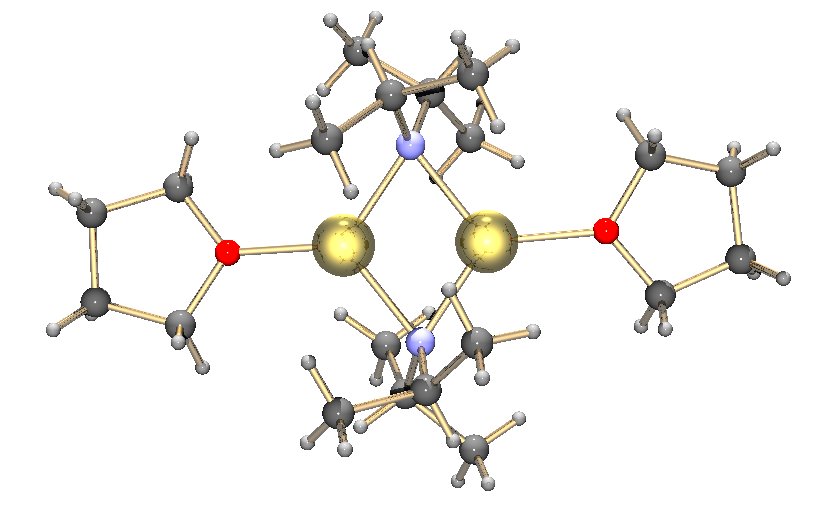
Aldol–Tishchenko Reaction
The Aldol–Tishchenko reaction is a tandem reaction involving an aldol reaction and a Tishchenko reaction. In organic synthesis it is a method to convert aldehydes and ketones into 1,3- hydroxyl compounds. The reaction sequence in many examples starts from conversion of a ketone into an enolate by action of lithium diisopropylamide (LDA), to which a suitable aldehyde is added. The resulting mono-ester diol is then converted into the diol by a hydrolysis step. With both the acetyl trimethylsilane In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, ... and propiophenone as reactants, the diol is obtained as a pure diastereoisomer. References {{DEFAULTSORT:Aldol-Tishchenko reaction Addition reactions Name reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tandem Reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the previous step.Tietze, L. F.; Beifuss, U. ''Angew. Chem. Int. Ed.'' 1993, ''32'', 131–163. In cascade reactions, isolation of intermediates is not required, as each reaction composing the sequence occurs spontaneously. In the strictest definition of the term, the reaction conditions do not change among the consecutive steps of a cascade and no new reagents are added after the initial step.Padwa, A.; Bur, S. K. ''Tetrahedron'' 2007, ''63'', 5341–5378. By contrast, one-pot procedures similarly allow at least two reactions to be carried out consecutively without any isolation of intermediates, but do not preclude the addition of new reagents or the change of conditions after the first reaction. Thus, any cascade reaction is also a one-pot p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl Trimethylsilane
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, although this term is barely heard. The acetyl group contains a methyl group () single-bonded to a carbonyl (). The carbonyl center of an acyl radical has one nonbonded electron with which it forms a chemical bond to the remainder ''R'' of the molecule. The acetyl moiety is a component of many organic compounds, including acetic acid, the neurotransmitter acetylcholine, acetyl-CoA, acetylcysteine, acetaminophen (also known as paracetamol), and acetylsalicylic acid (also known as aspirin). Acetylation In nature The introduction of an acetyl group into a molecule is called acetylation. In biological organisms, acetyl groups are commonly transferred from acetyl-CoA to other organic molecules. Acetyl-CoA is an intermediate both in the bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Organic Chemistry
''The Journal of Organic Chemistry'', colloquially known as ''JOC'', is a peer-reviewed scientific journal for original contributions of fundamental research in all branches of theory and practice in organic and bioorganic chemistry. It is published by the publishing arm of the American Chemical Society, with 24 issues per year. According to the ''Journal Citation Reports'', the journal had a 2017 impact factor of 4.805 and it is the journal that received the most cites (100,091 in 2017) in the field of organic chemistry. According to Web of Knowledge (and as December 2012), eleven papers from the journal have received more than 1,000 citations, with the most cited paper having received 7,967 citations. The current editor-in-chief is Scott J. Miller from Yale University. Indexing ''J. Org. Chem.'' is currently indexed in: See also *Organic Letters *Organometallics ''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry Letters
''Chemistry Letters'' is a peer-reviewed scientific journal published by the Chemical Society of Japan. It specializes in the rapid publication of reviews and letters on all areas of chemistry. The editor-in-chief is Mitsuhiko Shionoya (University of Tokyo). According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 1.23. References External links * Chemistry journals Publications established in 1972 English-language journals Academic journals published by learned and professional societies Monthly journals Chemical Society of Japan {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereoisomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propiophenone
Propiophenone (shorthand: benzoylethane or BzEt) is an aryl ketone. It is a colorless, sweet-smelling liquid that is insoluble in water, but miscible with organic solvents. It is used in the preparation of other compounds. Production Propiophenone can be prepared by Friedel–Crafts reaction of propanoyl chloride and benzene. It is also prepared commercially by ketonization of benzoic acid and propionic acid over calcium acetate and alumina at 450–550 °C: :C6H5CO2H + CH3CH2CO2H → C6H5C(O)CH2CH3 + CO2 + H2O Ludwig Claisen Rainer Ludwig Claisen (; 14 January 1851 – 5 January 1930) was a German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the u ... discovered that α-methoxystyrene forms this compound when heated for an hour at 300 °C (65% yield). Uses 120px, left, appetite_suppressant.html" ;"title="Phenmetrazine, derived from propio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry. Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carbonyl compounds (the original experiments used aldehydes) to form a new β-hydroxy carbonyl compound. These products are known as '' aldols'', from the ''ald''ehyde + alcoh''ol'', a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic. For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritol and the synthesis of the heart disease drug Lipitor ( atorvastatin, calcium salt). The aldol reaction unites two relatively simple molecules into a more complex one. Increased complexity arises because up to two new stereogenic centers (on the α- and β-carbon of the aldol adduct, marked w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group. Preparation and structure LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of tetrahydrofuran and diisopropylamine with ''n''-butyllithium. When dissociated, the diisopropylamide anion can become protonated to form diisopropylamine. Diisopropylamine has a p''K''a value of 36. Therefore, its conjugate base is suitable for the deprotonation of compounds with greater acidity, importantly, such weakly acidic compounds (carbon acids) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds. Bonding and structure Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both an alkoxide and a carbanion. Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates. Preparation Deprotonation of enolizable ketones, aromatic alcohols, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA). Often, as in conventional Claisen condensations, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |