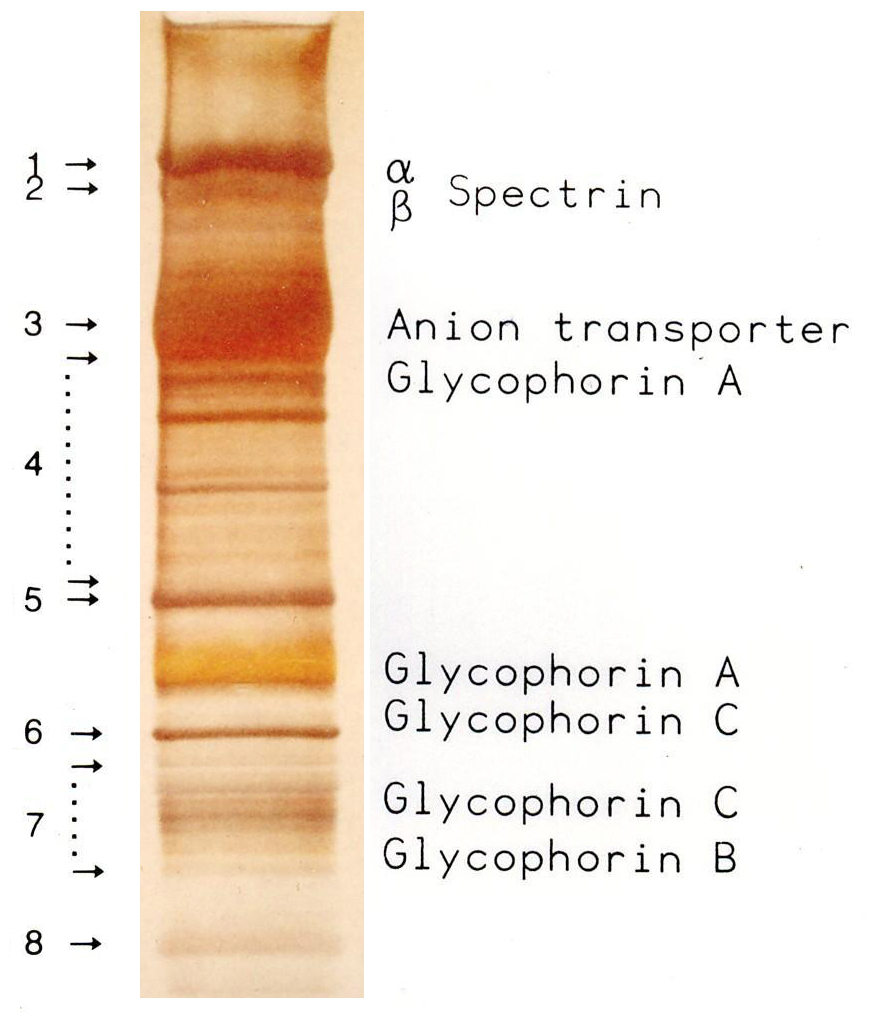
|
Acrydite
Acrydite is a phosphoramidite that allows the synthesis of oligonucleotides with a methacryl group at the 5' end (less commonly 3' or internal). Acryl oligonucleotides have been tested, but the acryl group is not stable to storage. Acrydite-modified oligonucleotides can react with nucleophiles such as thiols (Michael addition chemistry), this forms the basis of the ez-rays chemistry which was used for microarrays. More importantly, Acrydite-modified oligonucleotides can be incorporated, stoichiometrically, into hydrogels such as polyacrylamide, using standard free radical polymerization chemistry, where the double bond in the Acrydite group reacts with other activated double bond containing compounds such as acrylamide. History The idea of acrylamide-modified DNA was developed by T. Christian Boles, while working at Mosaic Technologies, a now-defunct biotechnology company located in Waltham, MA. The IP was licensed, along with a microarray technology ("ez-rays") to Matrix Technol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrydite
Acrydite is a phosphoramidite that allows the synthesis of oligonucleotides with a methacryl group at the 5' end (less commonly 3' or internal). Acryl oligonucleotides have been tested, but the acryl group is not stable to storage. Acrydite-modified oligonucleotides can react with nucleophiles such as thiols (Michael addition chemistry), this forms the basis of the ez-rays chemistry which was used for microarrays. More importantly, Acrydite-modified oligonucleotides can be incorporated, stoichiometrically, into hydrogels such as polyacrylamide, using standard free radical polymerization chemistry, where the double bond in the Acrydite group reacts with other activated double bond containing compounds such as acrylamide. History The idea of acrylamide-modified DNA was developed by T. Christian Boles, while working at Mosaic Technologies, a now-defunct biotechnology company located in Waltham, MA. The IP was licensed, along with a microarray technology ("ez-rays") to Matrix Technol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylamide
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primary amide (CONH2). It is produced industrially mainly as a precursor to polyacrylamides, which find many uses as water-soluble thickeners and flocculation agents. Acrylamide forms in burnt areas of food, particularly starchy foods like potatoes, when cooked with high heat, above . Acrylamide is highly toxic, linked to cancer in animal testing though not likely to be carcinogenic for humans, but its main derivative polyacrylamide is nontoxic. The possibility that this innocuous polymer contains traces of its hazardous precursor has long attracted attention. Because acrylamide is volatile and hazardous, it is mainly handled as an aqueous solution. Production Acrylamide can be prepared by the hydration of acrylonitrile: :CH2=CHCN + H2O → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Science (journal)
''Science'', also widely referred to as ''Science Magazine'', is the peer-reviewed academic journal of the American Association for the Advancement of Science (AAAS) and one of the world's top academic journals. It was first published in 1880, is currently circulated weekly and has a subscriber base of around 130,000. Because institutional subscriptions and online access serve a larger audience, its estimated readership is over 400,000 people. ''Science'' is based in Washington, D.C., United States, with a second office in Cambridge, UK. Contents The major focus of the journal is publishing important original scientific research and research reviews, but ''Science'' also publishes science-related news, opinions on science policy and other matters of interest to scientists and others who are concerned with the wide implications of science and technology. Unlike most scientific journals, which focus on a specific field, ''Science'' and its rival ''Nature (journal), Nature'' c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Computing
DNA computing is an emerging branch of unconventional computing which uses DNA, biochemistry, and molecular biology hardware, instead of the traditional electronic computing. Research and development in this area concerns theory, experiments, and applications of DNA computing. Although the field originally started with the demonstration of a computing application by Len Adleman in 1994, it has now been expanded to several other avenues such as the development of storage technologies, nanoscale imaging modalities, synthetic controllers and reaction networks, etc. A brief history of DNA computing and molecular programming Leonard Adleman of the University of Southern California initially developed this field in 1994. — The first DNA computing paper. Describes a solution for the directed Hamiltonian path problem. Also available here: Adleman demonstrated a proof-of-concept use of DNA as a form of computation which solved the seven-point Hamiltonian path problem. Since the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Satisfiability Problem
In mathematical logic, a formula is ''satisfiable'' if it is true under some assignment of values to its variables. For example, the formula x+3=y is satisfiable because it is true when x=3 and y=6, while the formula x+1=x is not satisfiable over the integers. The dual concept to satisfiability is validity; a formula is ''valid'' if every assignment of values to its variables makes the formula true. For example, x+3=3+x is valid over the integers, but x+3=y is not. Formally, satisfiability is studied with respect to a fixed logic defining the syntax of allowed symbols, such as first-order logic, second-order logic or propositional logic. Rather than being syntactic, however, satisfiability is a semantic property because it relates to the ''meaning'' of the symbols, for example, the meaning of + in a formula such as x+1=x. Formally, we define an interpretation (or model) to be an assignment of values to the variables and an assignment of meaning to all other non-logical symbols, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecularly Imprinted Polymer
A molecularly imprinted polymer (MIP) is a polymer that has been processed using the molecular imprinting technique which leaves cavities in the polymer matrix with an affinity for a chosen "template" molecule. The process usually involves initiating the polymerization of monomers in the presence of a template molecule that is extracted afterwards, leaving behind complementary cavities. These polymers have affinity for the original molecule and have been used in applications such as chemical separations, catalysis, or molecular sensors. Published works on the topic date to the 1930s. Molecular imprinting techniques (state of the art and perspectives) Molecular imprinting is the process of generating an impression within a solid or a gel, the size, shape and charge distribution of which corresponds to a template molecule (typically present during polymerisation). The result is a synthetic receptor capable of binding to a target molecule, which fits into the binding site with high affi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aptamer
Aptamers are short sequences of artificial DNA, RNA, XNA, or peptide that bind a specific target molecule, or family of target molecules. They exhibit a range of affinities ( KD in the pM to μM range), with little or no off-target binding and are sometimes classified as chemical antibodies. Aptamers and antibodies can be used in many of the same applications, but the nucleic acid-based structure of aptamers, which are mostly oligonucleotides, is very different from the amino acid-based structure of antibodies, which are proteins. This difference can make aptamers a better choice than antibodies for some purposes (see antibody replacement). Aptamers are used in biological lab research and medical tests. If multiple aptamers are combined into a single assay, they can measure large numbers of different proteins in a sample. They can be used to identify molecular markers of disease, or can function as drugs, drug delivery systems and controlled drug release systems. They a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Persistence Length
The persistence length is a basic mechanical property quantifying the bending stiffness of a polymer. The molecule behaves like a flexible elastic rod/beam (beam theory). Informally, for pieces of the polymer that are shorter than the persistence length, the molecule behaves like a rigid rod, while for pieces of the polymer that are much longer than the persistence length, the properties can only be described statistically, like a three-dimensional random walk. Formally, the persistence length, ''P'', is defined as the length over which correlations in the direction of the tangent are lost. In a more chemical based manner it can also be defined as the average sum of the projections of all bonds j ≥ i on bond i in an infinitely long chain. Let us define the angle ''θ'' between a vector that is tangent to the polymer at position 0 (zero) and a tangent vector at a distance ''L'' away from position 0, along the contour of the chain. It can be shown that the expectation value o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerase Chain Reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) to a large enough amount to study in detail. PCR was invented in 1983 by the American biochemist Kary Mullis at Cetus Corporation; Mullis and biochemist Michael Smith (chemist), Michael Smith, who had developed other essential ways of manipulating DNA, were jointly awarded the Nobel Prize in Chemistry in 1993. PCR is fundamental to many of the procedures used in genetic testing and research, including analysis of Ancient DNA, ancient samples of DNA and identification of infectious agents. Using PCR, copies of very small amounts of DNA sequences are exponentially amplified in a series of cycles of temperature changes. PCR is now a common and often indispensable technique used in medical laboratory research for a broad variety of applications ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain. Free-radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of free-radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by free-radical polymerization. Free-radical polymerization is a type of chain-growth polymeriz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
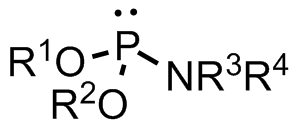
Phosphoramidite
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety. Applications Nucleoside phosphoramidites Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs. As ligands Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis. A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondary amines. This type of ligand was first used in 1996 in an asymmetric copper-catalysed addition of dialkylzincs to enones. See also * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |