|
Acetoxolone
Acetoxolone (also known as acetylglycyrrhetic acid, acetylglycyrrhetinic acid, glycyrrhetinyl acetate and glycyrrhetic acid acetate) is a drug used for peptic ulcer and gastroesophageal reflux disease. It is an acetyl derivative of glycyrrhetinic acid. It is found in Echinopora lamellosa. See also * Carbenoxolone Carbenoxolone (CBX) is a glycyrrhetinic acid derivative with a steroid-like structure, similar to substances found in the root of the licorice plant. Carbenoxolone is used for the treatment of peptic, esophageal and oral ulceration and inflammat ... * Enoxolone References 11β-Hydroxysteroid dehydrogenase inhibitors Drugs acting on the gastrointestinal system and metabolism Triterpenes Carboxylic acids Ketones Acetate esters {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enoxolone
Enoxolone (INN, BAN; also known as glycyrrhetinic acid or glycyrrhetic acid) is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice. It is used in flavoring and it masks the bitter taste of drugs like aloe and quinine. It is effective in the treatment of peptic ulcer and also has expectorant (antitussive) properties. It has some additional pharmacological properties with possible antiviral, antifungal, antiprotozoal, and antibacterial activities. Mechanism of action Glycyrrhetinic acid inhibits the enzymes (15-hydroxyprostaglandin dehydrogenase and delta-13-prostaglandin) that metabolize the prostaglandins PGE-2 and PGF-2α to their respective, inactive 15-keto-13,14-dihydro metabolites. This increases prostaglandins in the digestive system. Prostaglandins inhibit gastric secretion, stimulate pancreatic secretion and mucous secretion in the intestines, and markedly increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycyrrhetinic Acid
Enoxolone (International Nonproprietary Name, INN, British Approved Name, BAN; also known as glycyrrhetinic acid or glycyrrhetic acid) is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice. It is used in flavoring and it masks the bitter taste of drugs like aloe and quinine. It is effective in the treatment of peptic ulcer and also has cough medicine, expectorant (antitussive) properties. It has some additional pharmacological properties with possible antiviral, antifungal, antiprotozoal, and antibacterial activities. Mechanism of action Glycyrrhetinic acid inhibits the enzymes (15-hydroxyprostaglandin dehydrogenase (NAD+), 15-hydroxyprostaglandin dehydrogenase and delta-13-prostaglandin) that metabolize the prostaglandins PGE-2 and PGF-2α to their respective, inactive 15-keto-13,14-dihydro metabolites. This increases prostaglandins in the digestive system. Prostaglandins inhi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
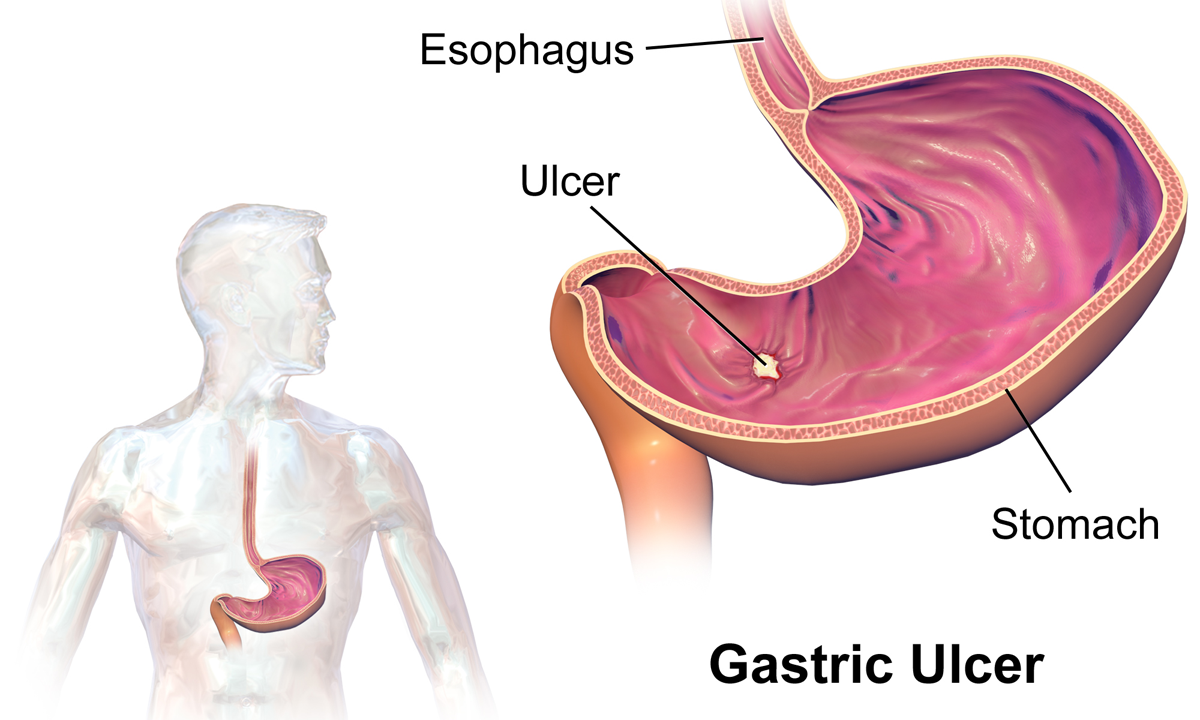
Peptic Ulcer
Peptic ulcer disease (PUD) is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines is a duodenal ulcer. The most common symptoms of a duodenal ulcer are waking at night with upper abdominal pain and upper abdominal pain that improves with eating. With a gastric ulcer, the pain may worsen with eating. The pain is often described as a burning or dull ache. Other symptoms include belching, vomiting, weight loss, or poor appetite. About a third of older people have no symptoms. Complications may include bleeding, perforation, and blockage of the stomach. Bleeding occurs in as many as 15% of cases. Common causes include the bacteria ''Helicobacter pylori'' and non-steroidal anti-inflammatory drugs (NSAIDs). Other, less common causes include tobacco smoking, stress as a result of other serious health conditions, Behçet's di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastroesophageal Reflux Disease
Gastroesophageal reflux disease (GERD) or gastro-oesophageal reflux disease (GORD) is one of the upper gastrointestinal chronic diseases where stomach content persistently and regularly flows up into the esophagus, resulting in symptoms and/or complications. Symptoms include dental corrosion, dysphagia, heartburn, odynophagia, regurgitation, non-cardiac chest pain, extraesophageal symptoms such as chronic cough, hoarseness, reflux-induced laryngitis, or asthma. On the long term, and when not treated, complications such as esophagitis, esophageal stricture, and Barrett's esophagus may arise. Risk factors include obesity, pregnancy, smoking, hiatal hernia, and taking certain medications. Medications that may cause or worsen the disease include benzodiazepines, calcium channel blockers, tricyclic antidepressants, NSAIDs, and certain asthma medicines. Acid reflux is due to poor closure of the lower esophageal sphincter, which is at the junction between the stomach and the esoph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, although this term is barely heard. The acetyl group contains a methyl group () single-bonded to a carbonyl (). The carbonyl center of an acyl radical has one nonbonded electron with which it forms a chemical bond to the remainder ''R'' of the molecule. The acetyl moiety is a component of many organic compounds, including acetic acid, the neurotransmitter acetylcholine, acetyl-CoA, acetylcysteine, acetaminophen (also known as paracetamol), and acetylsalicylic acid (also known as aspirin). Acetylation In nature The introduction of an acetyl group into a molecule is called acetylation. In biological organisms, acetyl groups are commonly transferred from acetyl-CoA to other organic molecules. Acetyl-CoA is an intermediate both ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Echinopora
''Echinopora'' is a genus of stony corals in the family Merulinidae. Species The following species are currently recognized: * ''Echinopora ashmorensis'' Veron, 1990 * ''Echinopora forskaliana'' (Milne Edwards & Haime, 1849) * ''Echinopora fruticulosa'' Klunzinger, 1879 * ''Echinopora gemmacea'' (Lamarck, 1816) * ''Echinopora hirsutissima'' Milne Edwards & Haime, 1849 * ''Echinopora horrida'' Dana, 1846 * ''Echinopora irregularis'' Veron, Turak & DeVantier, 2000 * ''Echinopora lamellosa'' (Esper, 1795) * ''Echinopora mammiformis'' (Nemenzo, 1959) * ''Echinopora pacificus'' Veron, 1990 * ''Echinopora robusta'' Veron, 2000 * ''Echinopora spinulosa'' Brüggemann * ''Echinopora tiranensis ''Echinopora'' is a genus of stony corals in the family Merulinidae. Species The following species In biology, a species is the basic unit of classification and a taxonomic rank of an organism, as well as a unit of biodiversity. A ...'' Veron, Turak & DeVantier, 2000 Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenoxolone
Carbenoxolone (CBX) is a glycyrrhetinic acid derivative with a steroid-like structure, similar to substances found in the root of the licorice plant. Carbenoxolone is used for the treatment of peptic, esophageal and oral ulceration and inflammation. Electrolyte imbalance is a serious side effect of carbenoxolone when used systemically. Carbenoxolone reversibly inhibits the conversion of inactive cortisone to cortisol by blocking 11β-hydroxysteroid dehydrogenase (11β-HSD). 11β-HSD also reversibly catalyzes the conversion of 7-ketocholesterol to 7-beta-hydroxycholesterol. Carbenoxolone is a modestly potent, reasonably effective, water-soluble blocker of gap junctions. Carbenoxolone has also been used in topical creams such as Carbosan gel, marketed for treatment of lip sores and mouth ulcers. Nootropic effects Carbenoxolone has also been investigated for nootropic effects. This research started from an observation that long-term exposure to glucocorticoids may have negative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drugs Acting On The Gastrointestinal System And Metabolism
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalation, injection, smoking, ingestion, absorption via a patch on the skin, suppository, or dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to treat, cure, prevent, or diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a regular basis for chronic disorders. Pharmaceutical drugs are often classified into drug classes—groups of related drugs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpenes
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' oft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered ret ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |