|
5-bromo-2'-deoxyuridine
Bromodeoxyuridine (5-bromo-2'-deoxyuridine, BrdU, BUdR, BrdUrd, broxuridine) is a synthetic nucleoside analogue with a chemical structure similar to thymidine. BrdU is commonly used to study cell proliferation in living tissues and has been studied as a radiosensitizer and diagnostic tool in people with cancer. During S phase of the cell cycle (when DNA replication occurs), BrdU can be incorporated in place of thymidine in newly synthesized DNA molecules of dividing cells. Cells that have recently performed DNA replication or DNA repair can be detected with antibodies specific for BrdU using techniques such as immunohistochemistry or immunofluorescence. BrdU-labelled cells in humans can be detected up to two years after BrdU infusion. Because BrdU can replace thymidine during DNA replication, it can cause mutations, and its use is therefore potentially a health hazard. However, because it is neither radioactive nor myelotoxic at labeling concentrations, it is widely preferred f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside Analogue
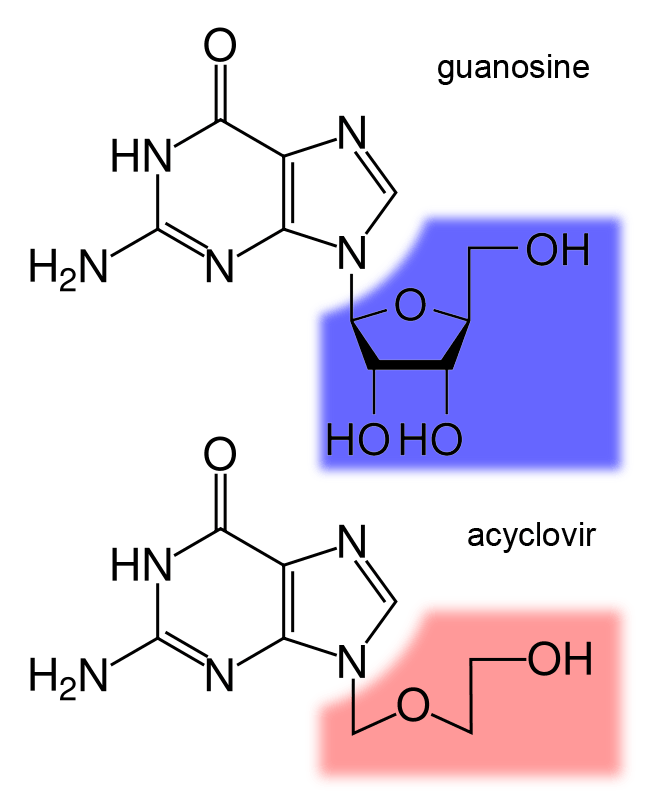
Nucleoside analogues are nucleosides which contain a nucleic acid analogue and a sugar. Nucleotide analogs are nucleotides which contain a nucleic acid analogue, a sugar, and a phosphate group with one to three phosphates. Nucleoside and nucleotide analogues can be used in therapeutic drugs, including a range of antiviral products used to prevent viral replication in infected cells. The most commonly used is acyclovir, although its inclusion in this category is uncertain, because it acts as a nucleoside but contains no actual sugar, as the sugar ring is replaced by an open-chain structure. Nucleotide and nucleoside analogues can also be found naturally. Examples include ddhCTP (3ʹ-deoxy-3′,4ʹdidehydro-CTP) produced by the human antiviral protein viperin and sinefungin (a S-Adenosyl methionine analogue) produced by some ''Streptomyces''. Function These agents can be used against hepatitis B virus, hepatitis C virus, herpes simplex, and HIV. Once they are phosphorylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myelotoxic
Bone marrow suppression also known as myelotoxicity or myelosuppression, is the decrease in production of cells responsible for providing immunity (leukocytes), carrying oxygen (erythrocytes), and/or those responsible for normal blood clotting (thrombocytes). Bone marrow suppression is a serious side effect of chemotherapy and certain drugs affecting the immune system such as azathioprine. The risk is especially high in cytotoxic chemotherapy for leukemia. Nonsteroidal anti-inflammatory drugs (NSAIDs), in some rare instances, may also cause bone marrow suppression. The decrease in blood cell counts does not occur right at the start of chemotherapy because the drugs do not destroy the cells already in the bloodstream (these are not dividing rapidly). Instead, the drugs affect new blood cells that are being made by the bone marrow. When myelosuppression is severe, it is called myeloablation. Many other drugs including common antibiotics may cause bone marrow suppression. Unlike che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organobromides
Organobromine compounds, also called organobromides, are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application of synthetic organobromine compounds is the use of polybrominated diphenyl ethers as fire-retardants, and in fact fire-retardant manufacture is currently the major industrial use of the element bromine. A variety of minor organobromine compounds are found in nature, but none are biosynthesized or required by mammals. Organobromine compounds have fallen under increased scrutiny for their environmental impact. General properties Most organobromine compounds, like most organohalide compounds, are relatively nonpolar. Bromine is more electronegative than carbon (2.9 vs 2.5). Consequently, the carbon in a carbon–bromine bond is electrophilic, i.e. alkyl bromides are alkylating agents. Carbon–halogen bond strengths, or bond dissociation energies are of 115, 83.7, 72.1, and 57.6 kc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staining Dyes
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells), or organelles within individual cells. In biochemistry, it involves adding a class-specific ( DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis. Light microscopes are used for viewing st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleosides
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building-blocks of DNA and RNA. List of nucleosides and corresponding nucleobases The reason for 2 symbols, shorter and longer, is that the shorter ones are better for contexts where explicit disambiguation is superfluous (because context disambiguates) and the longer ones are for contexts where explicit disambiguation is judged to be needed or wise. For example, when discussing long nucleobase sequences in genomes, the CATG symbol system is much preferable to the Cyt-Ade-Thy-Gua symbol system (see '' N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genetics Techniques
Genetics is the study of genes, genetic variation, and heredity in organisms.Hartl D, Jones E (2005) It is an important branch in biology because heredity is vital to organisms' evolution. Gregor Mendel, a Moravian Augustinian friar working in the 19th century in Brno, was the first to study genetics scientifically. Mendel studied "trait inheritance", patterns in the way traits are handed down from parents to offspring over time. He observed that organisms (pea plants) inherit traits by way of discrete "units of inheritance". This term, still used today, is a somewhat ambiguous definition of what is referred to as a gene. Trait inheritance and molecular inheritance mechanisms of genes are still primary principles of genetics in the 21st century, but modern genetics has expanded to study the function and behavior of genes. Gene structure and function, variation, and distribution are studied within the context of the cell, the organism (e.g. dominance), and within the context o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypan Blue
Trypan blue is an azo dye. It is a direct dye for cotton textiles. In biosciences, it is used as a vital stain to selectively colour dead tissues or cells blue. Live cells or tissues with intact cell membranes are not coloured. Since cells are very selective in the compounds that pass through the membrane, in a viable cell trypan blue is not absorbed; however, it traverses the membrane in a dead cell. Hence, dead cells appear as a distinctive blue colour under a microscope. Since live cells are excluded from staining, this staining method is also described as a dye exclusion method. Background and chemistry Trypan blue is derived from toluidine, that is, any of several isomeric bases, C14H16N2, derived from toluene. Trypan blue is so-called because it can kill trypanosomes, the parasites that cause sleeping sickness. An analog of trypan blue, suramin, is used pharmacologically against trypanosomiasis. Trypan blue is also known as diamine blue and Niagara blue. The extinctio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Ethynyl-2'-deoxyuridine
5-Ethynyl-2′-deoxyuridine (EdU) is a thymidine analogue which is incorporated into the DNA of dividing cells. EdU is used to assay DNA synthesis in cell culture and detect cells in embryonic, neonatal and adult animals which have undergone DNA synthesis. Whilst at high doses it can be cytotoxic, this molecule is now widely used to track proliferating cells in multiple biological systems. EdU-labelling allows cells to be isolated without denaturing RNA, allowing researchers to determine the transcriptional profile of cells. This approach has been used to assess transcription in neuronal cells and tissues that have recently divided either ''in vitro'' or ''in vivo''. Detection EdU is detected with a fluorescent azide which forms a covalent bond using click chemistry. Unlike the commonly used bromodeoxyuridine, EdU detection requires no heat or acid treatment. EdU incorporated into DNA induces DNA damage, primarily during DNA replication, which is reflected by phosphorylation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Bromouridine
5-Bromouridine (abbreviated BrUrd, 5BrU, br5Urd or rarely the one letter code B) is a uridine derivative with a bromo substituent at the fifth carbon. BrUrd is incorporated into RNA and can be detected immunocytochemically and analysed by cytometry. It causes DNA damage through base substitution and increases the number of mutations. Uses Detecting half-Life activity 5-Bromouridine has also been used to detect the half-life of RNA molecules. In a method called 5'-bromo-uridine immunoprecipitation chase-deep sequencing analysis (BRIC-seq), 5'-bromouridine is used to label RNA and enable the measurement of RNA levels over time. This method was used in Tani et al.'s study determining RNA half-lives to investigate the function of non-coding RNAs. Splicing of pre-mRNA 5-Bromouridine was also used to study ''in vitro'' splicing of pre-mRNA in a study by Wansink. 5-Bromoruridine 5'-triphosphate (BruUTP) was used to label pre-mRNA and investigate the efficiency of splicing. They found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine
Guanine () ( symbol G or Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds. This unsaturated arrangement means the bicyclic molecule is planar. Properties Guanine, along with adenine and cytosine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA, and uracil only in RNA. Guanine has two tautomeric forms, the major keto form (see figures) and rare enol form. It binds to cytosine through three hydrogen bonds. In cytosine, the amino group acts as the hydrogen bond donor and the C-2 carbonyl and the N-3 amine as the hydrogen-bond acceptors. Guanine has the C-6 carbonyl group that acts as the hydrogen bond acceptor, while a group at N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomorphism (crystallography)
In chemistry isomorphism has meanings both at the level of crystallography and at a molecular level. In crystallography, compounds are isomorphous if their symmetry is the same and their unit cell parameters are similar Molecules are isomorphous if they have similar shapes. The coordination complexes tris(acetylacetonato)iron (Fe(acac)3) and tris(acetylacetonato)aluminium (Al(acac)3) are isomorphous. These compounds, both of ''D''3 symmetry have very similar shapes, as determined by bond lengths and bond angles. Isomorphous compounds give rise to isomorphous crystals and form solid solutions. Historically, crystal shape was defined by measuring the angles between crystal faces with a goniometer. Whereas crystals of Fe(acac)3 are deep red and crystals of Al(acac)3 are colorless, a solid solution of the two, i.e. Fe1−xAlx(acac)3 will be deep or pale pink depending on the Fe/Al ratio, x. Double sulfates, such as Tutton's salt, with the generic formula MI2MII(SO4)2.6H2O, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |