|
4-HO-MALT
4-HO-MALT (4-hydroxy-''N''-methyl-''N''-allyltryptamine) is a tryptamine derivative which has been sold as a designer drug, first being detected in Slovenia in 2021. See also * 4-HO-MiPT * 4-HO-McPT * 4-HO-MPT * 4-AcO-DALT * 5-MeO-MALT 5-MeO-MALT (5-methoxy-''N''-methyl-''N''-allyltryptamine) is a lesser-known psychedelic drug that is closely related to 5-MeO-DALT and has been sold online as a designer drug. Legality 5-MeO-MALT is illegal in Hungary. Sweden's public health a ... References Phenols Tryptamines Tertiary amines {{pharm-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamines
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuasca brews. Many synthetic tryptamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-MALT
5-MeO-MALT (5-methoxy-''N''-methyl-''N''-allyltryptamine) is a lesser-known psychedelic drug that is closely related to 5-MeO-DALT and has been sold online as a designer drug. Legality 5-MeO-MALT is illegal in Hungary. Sweden's public health agency suggested classifying 5-MeO-MALT as a hazardous substance, on May 15, 2019. See also * 4-HO-MALT * 5-MeO-DET * 5-MeO-DiPT * 5-MeO-DMT * 5-MeO-DPT * 5-MeO-EiPT * 5-MeO-MiPT 5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs 5-MeO-DiPT, DiPT, and MiPT. It is commonly used as a "substitute" for 5-MeO-DiPT because o ... References Designer drugs Phenols Psychedelic tryptamines Serotonin receptor agonists {{hallucinogen-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
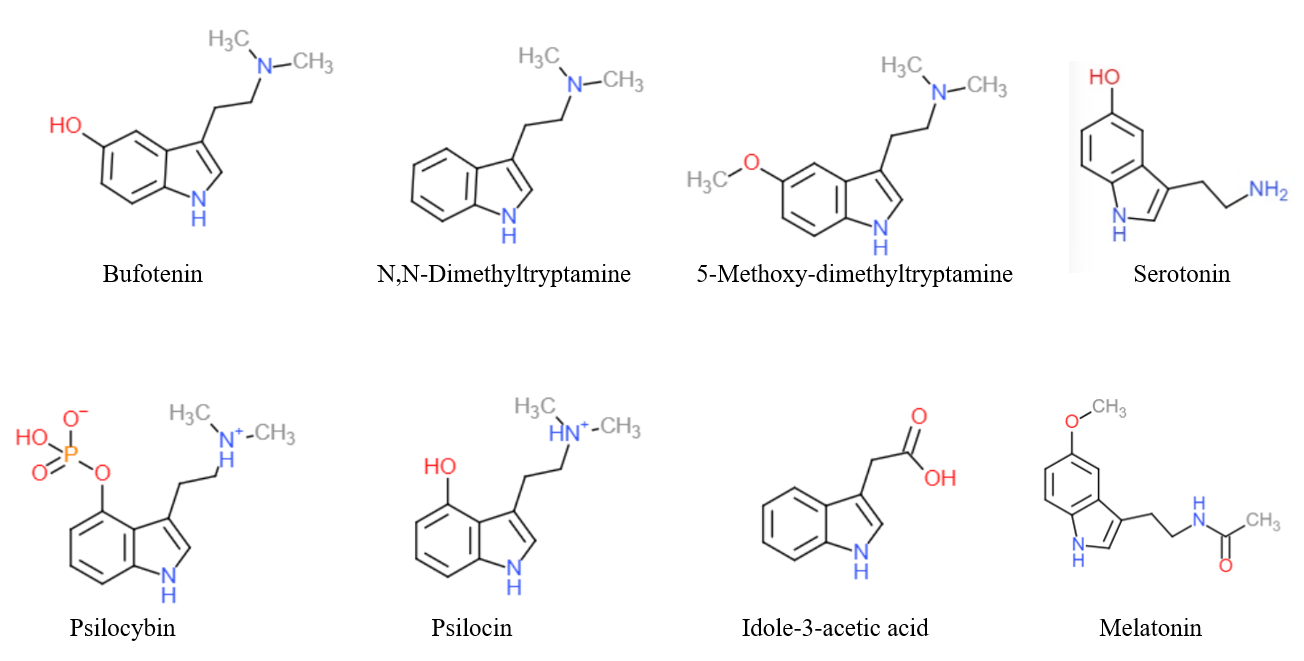
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drug
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-HO-MiPT
4-HO-MiPT (miprocin, 4-hydroxy-''N''-methyl-''N''-isopropyltryptamine) is a synthetic substituted aromatic compound and a lesser-known psychedelic tryptamine. It is thought to be a serotonergic psychedelic, similar to magic mushrooms, LSD and mescaline. Its molecular structure and pharmacological effects somewhat resemble those of the tryptamine psilocin, which is the primary psychoactive chemical in magic mushrooms. History 4-HO-MiPT was presumably first synthesized by Alexander Shulgin. Its synthesis is described in his book ''TiHKAL'' along with reports by people who had ingested the compound. Shulgin's trials and other anecdotal information suggest that 4-HO-MiPT is a synthetic psychedelic similar in activity to psilocin. It is relatively uncommon and has only a short history of human use. Chemistry Miprocin is the 4-hydroxyl analog of the chemical ''N''-methyl-''N''-isopropyltryptamine as well as the isopropyl homolog and possible structural analog of 4-HO-DMT. In Aug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-HO-MPT
4-Hydroxy-''N''-methyl-''N''-propyltryptamine, commonly known as 4-HO-MPT or meprocin, is a psychedelic drug in the tryptamine class of chemical compounds and is a higher homologue of the naturally occurring substituted tryptamine psilocin as well as being the 4-hydroxyl analog of MPT. History 4-HO-MPT was first synthesized and bioassayed by biochemist Alexander Shulgin and written about in his 1994 book ''TiHKAL''. Dosage and duration For psychedelic effects, the dosage and duration are listed as "unknown" in TiHKAL. Effects Very little data exists about the pharmacological properties, metabolism, and toxicity of 4-HO-MPT. In a single trial of 8 mg orally of 4-HO-MPT HCl from TiHKAL, it is described as producing visual distortion, vertigo, and slight insomnia. Legal status 4-HO-MPT is not scheduled by the United Nations' Convention on Psychotropic Substances. United States 4-HO-MPT is not scheduled at the federal level in the United States, but it is possible that 4- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-AcO-DALT
4-Acetyloxy-''N,N''-diallyltryptamine (or 4-AcO-DALT) is a tryptamine derivative. It has been sold as a designer drug, but little other information is available. It was first officially identified in seized drug samples in 2012. See also * DALT * 5-MeO-DALT 5-MeO-DALT or ''N,N''- di allyl-5-methoxy tryptamine is a psychedelic tryptamine first synthesized by Alexander Shulgin. Chemistry The full name of the chemical is ''N''-allyl-''N''-indol-3-yl)ethyl group">ethyl] propene, prop-2-en-1- amine. It i ... References Tryptamines Designer drugs Acetate esters Allylamines {{Pharm-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |