|
2-nitrophenyloctyl Ether
1-(2-Nitrophenoxy)octane, also known as nitrophenyl octyl ether and abbreviated NPOE, is a chemical compound that is used as a matrix in fast atom bombardment mass spectrometry, liquid secondary ion mass spectrometry, and as a highly lipophilic plasticizer in polymer membranes used in ion selective electrodes. See also * Glycerol * 3-Mercaptopropane-1,2-diol * 3-Nitrobenzyl alcohol * 18-Crown-6 * Sulfolane * Diethanolamine * Triethanolamine Triethanolamine, or TEA is a viscous organic compound that is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although samples m ... References {{DEFAULTSORT:Nitrophenoxy)octane, 1-(2- Nitrobenzenes Phenol ethers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethanolamine
Triethanolamine, or TEA is a viscous organic compound that is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although samples may appear yellow because of impurities. Production Triethanolamine is produced from the reaction of ethylene oxide with aqueous ammonia, also produced are ethanolamine and diethanolamine. The ratio of the products can be controlled by changing the stoichiometry of the reactants. : Applications Triethanolamine is used primarily in making surfactants, such as for emulsifier. It is a common ingredient in formulations used for both industrial and consumer products. The triethanolamine neutralizes fatty acids, adjusts and pH buffer, buffers the pH, and solubilizes oils and other ingredients that are not completely Solubility, soluble in water. Triethanolammonium salts in some cases are more soluble than salts of alkali metals that might be us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethanolamine
Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encountered as a colorless, viscous liquid. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" ( Group 2B). Production The reaction of ethylene oxide with aqueous ammonia first produces ethanolamine: :C2H4O + NH3 → H2NCH2CH2OH which reacts with a second and third equivalent of ethylene oxide to give DEA and triethanolamine: :C2H4O + H2NCH2CH2OH → HN(CH2CH2OH)2 :C2H4O + H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
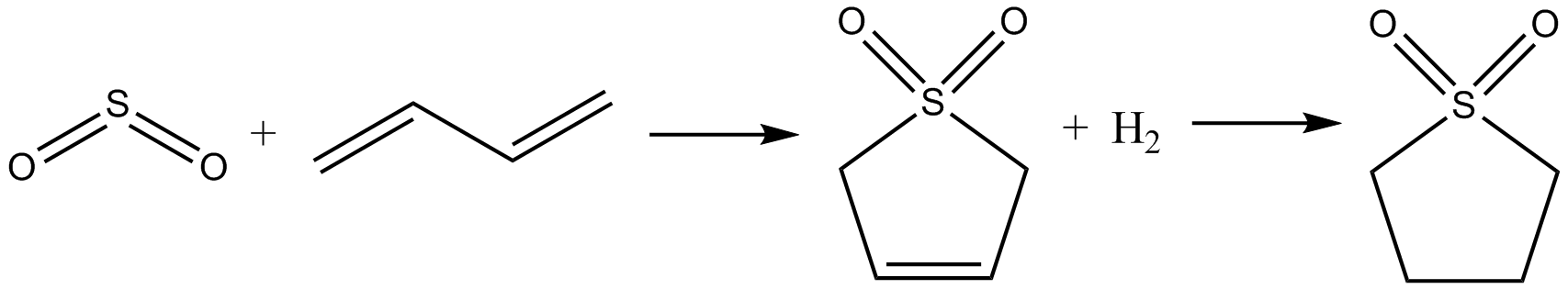
Sulfolane
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solvent for extractive distillation and chemical reactions. Sulfolane was originally developed by the Shell Oil Company in the 1960s as a solvent to purify butadiene. Sulfolane is a polar aprotic solvent, and it is readily soluble in water. Properties Sulfolane is classified as a sulfone, a group of organosulfur compounds containing a sulfonyl functional group. The sulfone group is a sulfur atom doubly bonded to two oxygen atoms and singly bonded to two carbon centers. The sulfur-oxygen double bond is polar, conferring good solubility in water, while the four carbon ring provides non-polar stability. These properties allow it to be miscible in both water and hydrocarbons, resulting in its widespread use as a solvent for purifying hydrocarbon mixtu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-Crown-6
18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is in cyclohexane and in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen. Synthesis This compound is prepared by a modified Williamson ether synthesis in the presence of a templating cation: It can be also prepared by the oligomerization of ethylene oxide: :(CH2OCH2CH2Cl)2 + (CH2OCH2CH2OH)2 + 2 KOH → (CH2CH2O)6 + 2 KCl + 2 H2O It can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Mercaptopropane-1,2-diol
3-Mercaptopropane-1,2-diol, also known as thioglycerol, is a chemical compound and thiol that is used as a matrix in fast atom bombardment mass spectrometry and liquid secondary ion mass spectrometry. See also * Glycerol * Mercaptoethanol 2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by sc ... References {{DEFAULTSORT:Mercaptopropane-1,2-diol, 3- Solvents Thiols Vicinal diols Mass spectrometry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Selective Electrode
An ion-selective electrode (ISE), also known as a specific ion electrode (SIE), is a transducer (or sensor) that converts the activity (chemistry), activity of a specific ion dissolved in a Solution (chemistry), solution into an electrical potential. The voltage is theoretically dependent on the logarithm of the ionic activity, according to the Nernst equation. Ion-selective electrodes are used in analytical chemistry and biochemistry, biochemical/biophysics, biophysical research, where measurements of ionic concentration in an aqueous solution are required. Types of ion-selective membrane There are four main types of ion-selective membrane used in ion-selective electrodes (ISEs): glass, solid state, liquid based, and compound electrode. Glass membranes Glass membranes are made from an ion-exchange type of glass (silicate or chalcogenide). This type of ISE has good Binding selectivity, selectivity, but only for several single-charged cations; mainly H+, Na+, and Ag+. Chalcogenid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
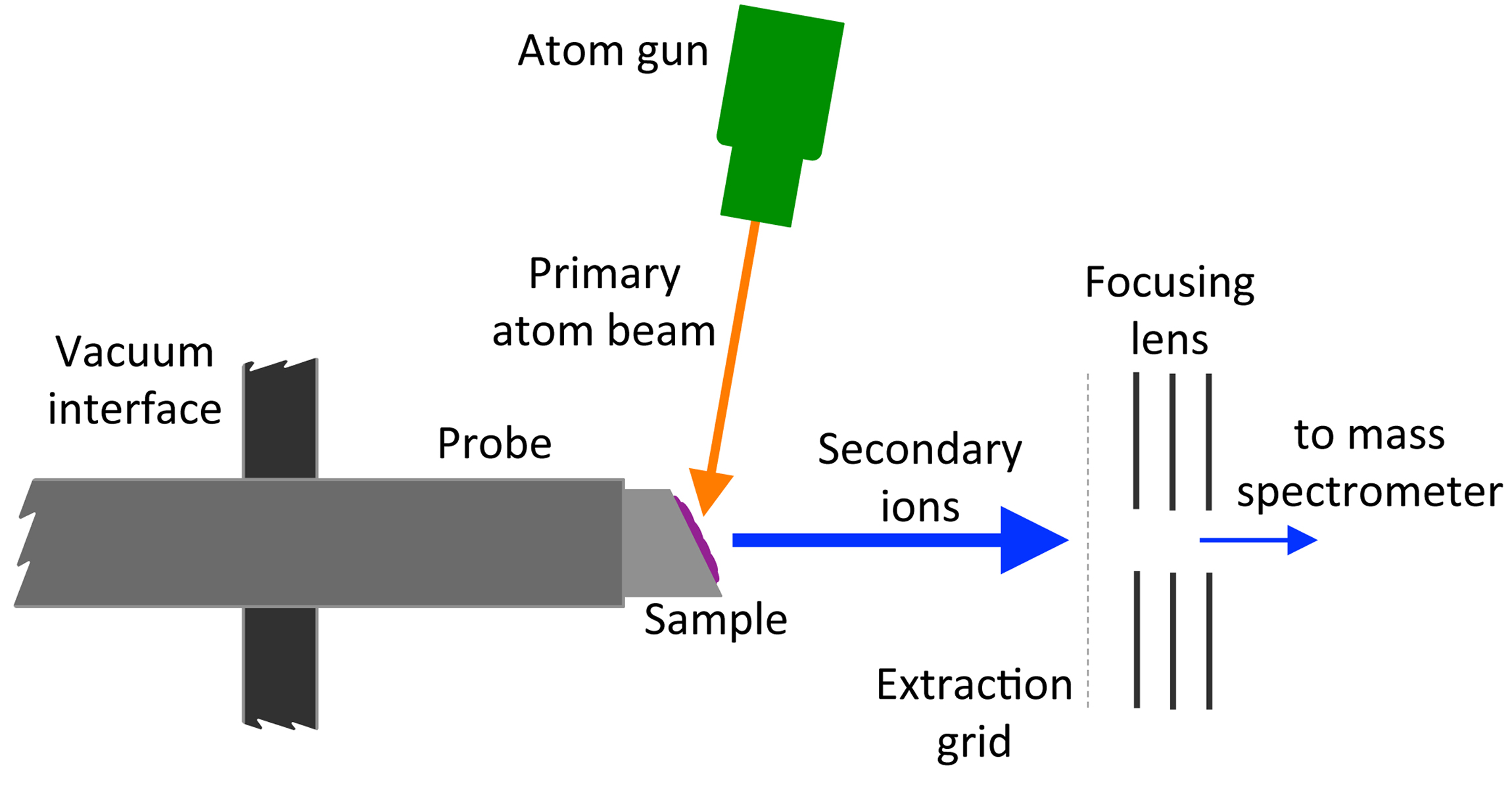
Fast Atom Bombardment
Fast atom bombardment (FAB) is an ionization technique used in mass spectrometry in which a beam of high energy atoms strikes a surface to create ions. It was developed by Michael Barber at the University of Manchester in 1980. When a beam of high energy ions is used instead of atoms (as in secondary ion mass spectrometry), the method is known as liquid secondary ion mass spectrometry (LSIMS). In FAB and LSIMS, the material to be analyzed is mixed with a non-volatile chemical protection environment, called a matrix, and is bombarded under vacuum with a high energy (4000 to 10,000 electron volts) beam of atoms. The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry. Ionization mechanism FAB is a rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymeric Membrane
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century.Pinnau, I., Freeman, B.D., ''Membrane Formation and Modification'', ACS, 1999. A wide variety of synthetic membranes is known.Osada, Y., Nakagawa, T., ''Membrane Science and Technology'', New York: Marcel Dekker, Inc,1992. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. The most of commercially utilized synthetic membranes in separation industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as a choice of driving force define a particular membrane sep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)
