|
2-Methoxyethanol
2-Methoxyethanol, or methyl cellosolve, is an organic compound with formula that is used mainly as a solvent. It is a clear, colorless liquid with an ether-like odor. It is in a class of solvents known as glycol ethers which are notable for their ability to dissolve a variety of different types of chemical compounds and for their miscibility with water and other solvents. It can be formed by the nucleophilic attack of methanol on protonated ethylene oxide followed by proton transfer: : + → + 2-Methoxyethanol is used as a solvent for many different purposes such as varnishes, dyes, and resins. It is also used as an additive in airplane deicing solutions. In organometallic chemistry it is commonly used for the synthesis of Vaska's complex and related compounds such as carbonylchlorohydridotris(triphenylphosphine)ruthenium (II). During these reactions the alcohol acts as a source of hydride and carbon monoxide. 2-Methoxyethanol is toxic to the bone marrow and testicles. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycol Ethers
Glycol ethers are a class of chemical compounds consisting of alkyl ethers that are based on glycols such as ethylene glycol or propylene glycol. They are commonly used as solvents in paints and cleaners. They have good solvent properties while having higher boiling points than the lower-molecular-weight ethers and alcohols. The name "Cellosolve" was registered in 1924 as a United States trademark by Carbide & Carbon Chemicals Corporation (a division of Union Carbide Corporation) for "Solvents for Gums, Resins, Cellulose Esters, and the Like". "Ethyl Cellosolve" or simply "Cellosolve" consists mainly of ethylene glycol monoethyl ether and was introduced as a lower-cost solvenet alternative to ethyl lactate. "Butyl Cellosolve" (ethylene glycol monobutyl ether) was introduced in 1928, and "Methyl Cellosolve" ( ethylene glycol monomethyl ether) in 1929. Glycol ethers are designated "E-series" for or "P-series" for those made from ethylene oxide or propylene oxide, respectively. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycol Ethers
Glycol ethers are a class of chemical compounds consisting of alkyl ethers that are based on glycols such as ethylene glycol or propylene glycol. They are commonly used as solvents in paints and cleaners. They have good solvent properties while having higher boiling points than the lower-molecular-weight ethers and alcohols. The name "Cellosolve" was registered in 1924 as a United States trademark by Carbide & Carbon Chemicals Corporation (a division of Union Carbide Corporation) for "Solvents for Gums, Resins, Cellulose Esters, and the Like". "Ethyl Cellosolve" or simply "Cellosolve" consists mainly of ethylene glycol monoethyl ether and was introduced as a lower-cost solvenet alternative to ethyl lactate. "Butyl Cellosolve" (ethylene glycol monobutyl ether) was introduced in 1928, and "Methyl Cellosolve" ( ethylene glycol monomethyl ether) in 1929. Glycol ethers are designated "E-series" for or "P-series" for those made from ethylene oxide or propylene oxide, respectively. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vaska's Complex
Vaska's complex is the trivial name for the chemical compound ''trans''-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO) (C6H5)3sub>2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually ''trans'' triphenylphosphine ligands, carbon monoxide and a chloride ion. The complex was first reported by J. W. DiLuzio and Lauri Vaska in 1961. Vaska's complex can undergo oxidative addition and is notable for its ability to bind to O2 reversibly. It is a bright yellow crystalline solid. Preparation The synthesis involves heating virtually any iridium chloride salt with triphenylphosphine and a carbon monoxide source. The most popular method uses dimethylformamide (DMF) as a solvent, and sometimes aniline is added to accelerate the reaction. Another popular solvent is 2-methoxyethanol. The reaction is typically conducted under nitrogen. In the synthesis, triphenylphosphine serves as both a ligand and a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krebs Cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized. The name of this metabolic pathway is derived from the citric acid (a tricarbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" also describes the conjugate acid, conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic ion, polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol Dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. In humans and many other animals, they serve to break down alcohols that otherwise are toxic, and they also participate in generation of useful aldehyde, ketone, or alcohol groups during biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+. Evolution Genetic evidence from comparisons of multiple organisms showed that a glutathione-dependent formaldehyde dehydrogenase, identical to a class III alcohol dehydrogenase (ADH-3/ADH5), is presumed to be the ancestral enzyme for the entire ADH family. Early on in evolution, an effective method for eliminating both endogenous and exogenous formaldehyde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
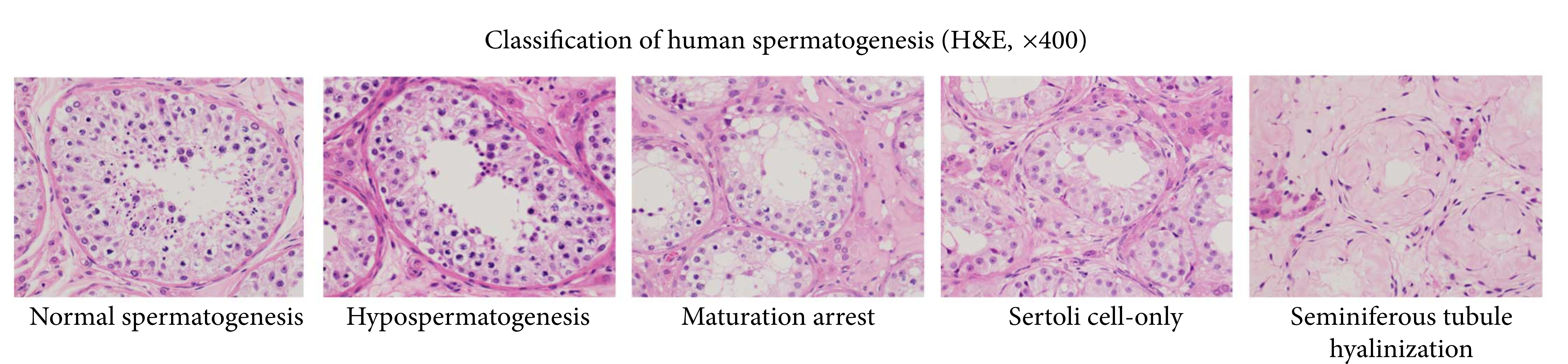
Azoospermia
Azoospermia is the medical condition of a man whose semen contains no sperm. It is associated with male infertility, but many forms are amenable to medical treatment. In humans, azoospermia affects about 1% of the male population and may be seen in up to 20% of male infertility situations in Canada. In a non-pathological context, azoospermia is also the intended result of a successful vasectomy. Classification Azoospermia can be classified into three major types as listed. Many conditions listed may also cause various degrees of oligospermia rather than azoospermia. Pretesticular and testicular azoospermia are known as non-obstructive azoospermia, whereas post-testicular azoospermia is considered obstructive. Pretesticular Pretesticular azoospermia is characterized by inadequate stimulation of otherwise normal testicles and genital tract. Typically, follicle-stimulating hormone (FSH) levels are low (hypogonadotropic) commensurate with inadequate stimulation of the testes to prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligospermia
Terms oligospermia, oligozoospermia, and low sperm count refer to semen with a low concentration of sperm and is a common finding in male infertility. Often semen with a decreased sperm concentration may also show significant abnormalities in sperm morphology and motility (technically oligoasthenoteratozoospermia). There has been interest in replacing the descriptive terms used in semen analysis with more quantitative information. Diagnosis The diagnosis of oligozoospermia is based on one low count in a semen analysis performed on two occasions. For many decades sperm concentrations of less than 20 million sperm/ml were considered low or oligospermic, recently, however, the WHO reassessed sperm criteria and established a lower reference point, less than 15 million sperm/ml, consistent with the 5th percentile for fertile men. Sperm concentrations fluctuate and oligospermia may be temporary or permanent. Sources usually classify oligospermia in 3 classes: *Mild: concentrations 10 mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |