|
2-Methylthiophene
2-Methylthiophene is an organosulfur compound with the formula CH3C4H3S. It is a colorless, flammable liquid. It can be produced by Wolff-Kishner reduction of thiophene-2-carboxaldehyde. Its commercial synthesis involvess vapor-phase dehydrogenation of a 1-pentanol/CS2 mixture.. See also *3-Methylthiophene 3-Methylthiophene is an organosulfur compound with the formula CH3C4H3S. It is a colorless, flammable liquid. It can be produced by sulfidation of 2-methylsuccinate. Like its isomer 2-methylthiophene, its commercial synthesis involvess vapor-p ... References {{DEFAULTSORT:Methylthiophene, 2- Thiophenes Methyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Methylthiophene
3-Methylthiophene is an organosulfur compound with the formula CH3C4H3S. It is a colorless, flammable liquid. It can be produced by sulfidation of 2-methylsuccinate. Like its isomer 2-methylthiophene, its commercial synthesis involvess vapor-phase dehydrogenation of suitable precursors. 3-Methylthiophene is a precursor to the drug thenyldiamine and the pesticide morantel Morantel is an anthelmintic drug used for the removal of parasitic worms in livestock. It affects the nervous system of worms given the drug is an inhibitor of acetylcholinesterase. It is derived in part from 3-methylthiophene. Morantel is close ..... References {{DEFAULTSORT:Methylthiophene, 3- Thiophenes Methyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two ( cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene-2-carboxaldehyde
Thiophene-2-carboxaldehyde is an organosulfur compound with the formula C4H3SCHO. It is one of two isomeric thiophenecarboxaldehydes. It is a colorless liquid that often appears amber after storage. It is versatile precursor to many drugs including eprosartan, Azosemide, and Teniposide. Preparation It can be prepared from thiophene Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ... by the Vilsmeier reaction. Alternatively, it is prepared from chloromethylation of thiophene. References {{reflist Thiophenes Aldehydes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
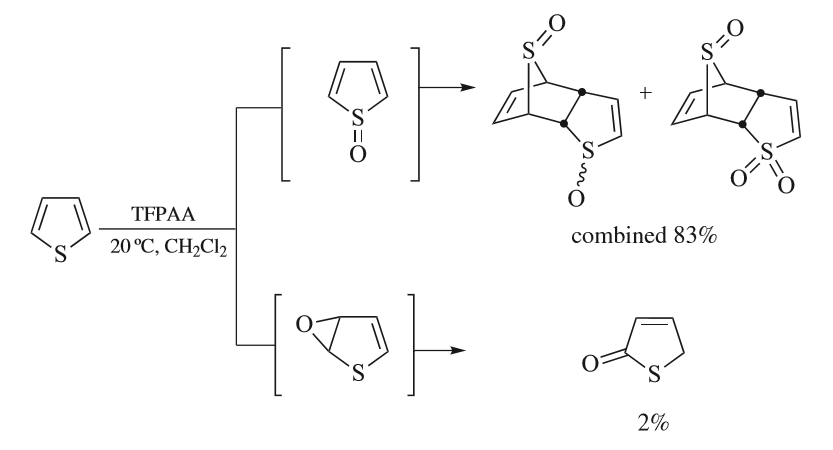
Thiophenes
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |