|
2,2′-Bipyridine
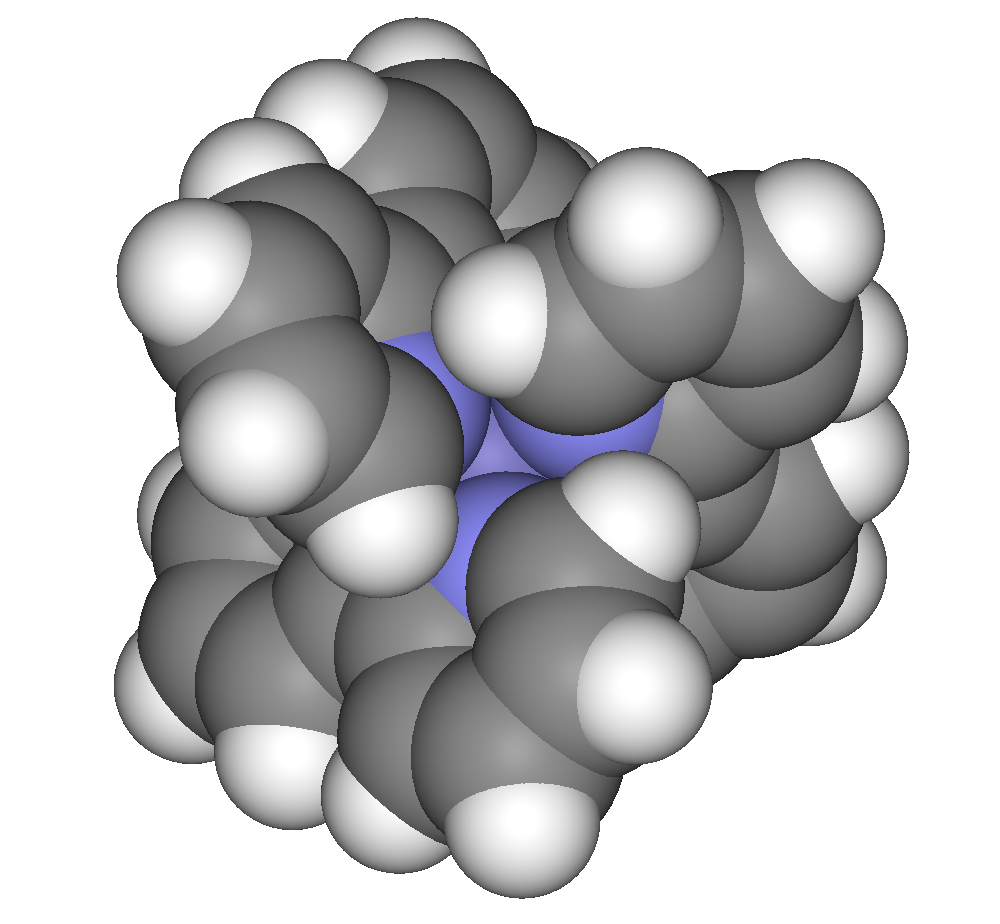
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications. Preparation, structure, and general properties 2,2'-Bipyridine was first prepared by decarboxylation of divalent metal derivatives of pyridine-2-carboxylate: :M(O2CC5H4N)2 → (C5H4N)2 + 2CO2 + ... It is prepared by the dehydrogenation of pyridine Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ... using Raney nickel: :2C5H5N → (C5H4N)2 + H2 Although bipyridine is often drawn with its nitrogen atoms in ''cis'' conformation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Complexes Of 2,2'-bipyridine
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties. Complexes Bipy forms a wide variety of complexes. Almost always, it is a bidentate ligand, binding metal centers with the two nitrogen atoms. Examples: *Mo(CO)4(bipy), derived from Mo(CO)6. * RuCl2(bipy)2, a popular precursor to mixed ligand complexes. * 2+.html" ;"title="u(bipy)3sup>2+">u(bipy)3sup>2+, a well studied luminophore. * e(bipy)3sup>2+ has been used for the colorimetric analysis of iron ions. Tris-bipy complexes Bipyridin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bipyridine
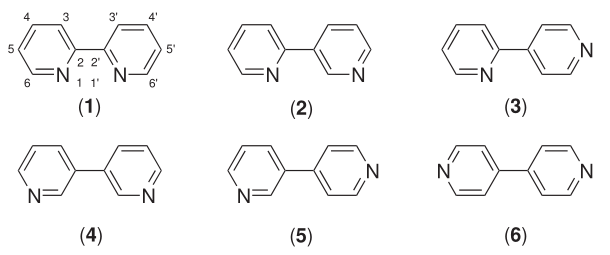
Bipyridines also known as bipyridyls, dipyridyls, and dipyridines, are a family of chemical compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. Bipyridines are of significance in pesticides. Six isomers of bipyridine exist, but two are prominent: 2,2′-bipyridine is a popular ligand. 4,4'-Bipyridine is a precursor to the commercial herbicide paraquat. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. 2,2′-Bipyridine 2,2′-Bipyridine (2,2′-bipy) is a chelating ligand that forms complexes with most transition metal ions that are of broad academic interest. Many of these complexes have distinctive optical properties, and some are of interest for analysis. Its complexes are used in studies of electron and energy transfer, supramolecular and materials chemistry, and catalysis. 2,2′-Bipyridine is used in the manufacture of diqua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2'-Biquinoline
2,2′-Biquinoline is an organic compound with the formula (C9H6N)2. It is one of several biquinolines. It is prepared by reductive coupling of 2-chloroquinoline. It is a colorimetric indicator for organolithium compounds. Ligand properties 2,2′-Biquinoline is a bidentate ligand. Unlike the related complexes of 2,2′-bipyridine 2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals ..., the metal does not typically occupy the plane of the biquinoline. References {{DEFAULTSORT:Biquinoline, 2, 2'- Quinolines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4,4'-Bipyridine
4,4′-Bipyridine (abbreviated to 4,4′-bipy or 4,4′-bpy) is an organic compound with the formula . It is one of several isomers of bipyridine. It is a colorless solid that is soluble in organic solvents. is mainly used as a precursor to ''N'',''N''′-dimethyl-4,4′-bipyridinium C5H4NCH3)2sup>2+, known as paraquat. History 4,4′-Bipyridine was first obtained in 1868 by the Scottish chemist Thomas Anderson via heating pyridine with sodium metal. However, Anderson's empirical formula for 4,4′-bipyridine was incorrect. The correct empirical formula, and the correct molecular structure, for 4,4′-bipyridine was provided in 1882 by the Austrian chemist Hugo Weidel and his student M. Russo. Uses 4,4'-Bipyridine is an intermediate in the production of paraquat, a widely-used herbicide. In this process, pyridine is oxidized to 4,4'-bipyridine in a coupling reaction, followed by dimethylation to form paraquat. : Reactions The reducing agent is N,N'-bis(trimethylsil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is given as charge times length of separation, it is a vector whose direction is in the direction of the unit vector of the position vector of the positive charge w.r.t negative charge: :p = ''q''r. named in honour of the physicist Peter J. W. Debye. It is defined as statcoulomb-centimeters.The statcoulomb is also known as the franklin or electrostatic unit of charge. :1 statC = 1 Fr = 1 esu = 1 cm3/2⋅g1/2⋅s−1. Historically the debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of 10−10 statcoulomb10−10 statcoulomb corresponds to approximately 0.2083 units of elementary charge. (generally called e.s.u. (electrostatic unit) in older scientific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg. It occurs in some nickel and copper ores along with some native deposits, mostly in South Africa, which accounts for ~80% of the world production. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable and is a major precious metal commodity. Platinum is one of the least reactive metals. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. Consequent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,10-Phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene. Abbreviated "phen", it is used as a ligand in coordination chemistry, forming strong complexes with most metal ions.Luman, C.R. and Castellano, F.N. (2003) "Phenanthroline Ligands" in Comprehensive Coordination Chemistry II. Elsevier. . It is often sold as the monohydrate. Synthesis Phenanthroline may be prepared by two successive Skraup reactions of glycerol with ''o''-phenylenediamine, catalyzed by sulfuric acid, and an oxidizing agent, traditionally aqueous arsenic acid or nitrobenzene. Dehydration of glycerol gives acrolein which condenses with the amine followed by a cyclization. Coordination chemistry In terms of its coordination properties, phenanthroline is similar to 2,2'-bipyridine (bipy) with the advantage that the two nitrogen donor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelate Ring
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the liquid phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At its simplest, it is useful way of converting alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Alkenes are precursors to aldehydes (), alcohols (), polymers, and aromatics. As a problematic reaction, the fouling and inactivation of many catalysts arises via coking, which is the dehydrogenative polymerization of organic substrates. Enzymes that catalyze dehydrogenation are called dehydrogenases. Heterogeneous catalytic routes Styrene Dehydrogenation processes are used extensively to produce aromatics in the petrochemical industry. Such processes are highly endothermic and require temperatures of 500 °C and above. Dehydrogenation also converts saturated fats to unsatura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |