|
2,2'-Biquinoline
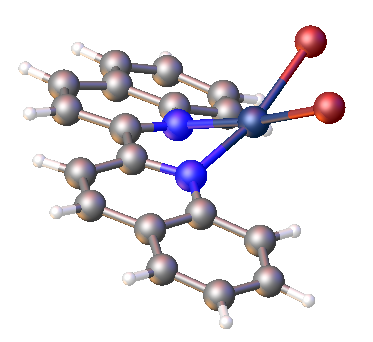
2,2′-Biquinoline is an organic compound with the formula (C9H6N)2. It is one of several biquinolines. It is prepared by reductive coupling of 2-chloroquinoline. It is a colorimetric indicator for organolithium compounds. Ligand properties 2,2′-Biquinoline is a bidentate ligand. Unlike the related complexes of 2,2′-bipyridine 2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals ..., the metal does not typically occupy the plane of the biquinoline. References {{DEFAULTSORT:Biquinoline, 2, 2'- Quinolines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2′-Bipyridine
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications. Preparation, structure, and general properties 2,2'-Bipyridine was first prepared by decarboxylation of divalent metal derivatives of pyridine-2-carboxylate: :M(O2CC5H4N)2 → (C5H4N)2 + 2CO2 + ... It is prepared by the dehydrogenation of pyridine Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ... using Raney nickel: :2C5H5N → (C5H4N)2 + H2 Although bipyridine is often drawn with its nitrogen atoms in ''cis'' conformation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Chloroquinoline
2-Chloroquinoline is an organic compound with the formula ClC9H6N. It is one of several isomeric chloro derivatives of the bicyclic heterocycle called quinoline. A white solid, 2-chloroquinoline can be prepared from vinylaniline and phosgene. It is a precursor to 2,2'-biquinoline 2,2′-Biquinoline is an organic compound with the formula (C9H6N)2. It is one of several biquinolines. It is prepared by reductive coupling of 2-chloroquinoline. It is a colorimetric indicator for organolithium compounds. Ligand properties .... References {{DEFAULTSORT:Chloroquinoline, 2- Quinolines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance. Occurrence and isolation Quinoline was first extracted from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge; he called quinoline ''leukol'' ("white oil" in Greek). Coal tar remains the principal source of commercial quinoline. In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, stry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bidentate Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



4-3D-balls.png)