|
2-Methoxyethyl-18-methoxycoronaridinate
(–)-2-Methoxyethyl-18-methoxycoronaridinate (ME-18-MC) is a second generation synthetic derivative of ibogaine developed by the research team led by the pharmacologist Stanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont. In animal studies it has shown similar efficacy to the related compound 18-methoxycoronaridine (18-MC) at reducing self-administration of morphine and methamphetamine but with higher potency by weight, showing anti-addictive effects at the equivalent of half the minimum effective dose of 18-MC. Similarly to 18-MC itself, ME-18-MC acts primarily as a selective α3β4 nicotinic acetylcholine antagonist, although it has a slightly stronger effect than 18-MC as an NMDA antagonist, and its effects on opioid receptors are weaker than those of 18-MC at all except the kappa opioid receptor, at which it has slightly higher affinity than 18-MC. See also * 18-Methylaminocoronaridine * 18-Methoxycoronarid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-Methylaminocoronaridine
(–)-18-Methylaminocoronaridine (18-MAC) is a second generation synthetic derivative of ibogaine developed by the research team led by the pharmacologist Stanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont. See also * 2-Methoxyethyl-18-methoxycoronaridinate * 18-Methoxycoronaridine * Coronaridine * Ibogaine * Noribogaine * Voacangine Voacangine (12-methoxyibogamine-18-carboxylic acid methyl ester) is an alkaloid found predominantly in the root bark of the '' Voacanga africana'' tree, as well as in other plants such as ''Tabernanthe iboga'', '' Tabernaemontana africana'', ''Tra ... References {{DEFAULTSORT:Methylaminocoronaridine, 18- Drug rehabilitation Iboga Nicotinic antagonists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-methoxycoronaridine
18-Methoxycoronaridine (18-MC, or MM-110) is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction. 18-MC was in the early stages of human testing by the California-based drug development company Savant HWP before being acquired by MindMed, a Canadian pharmaceutical company newly listed on the NASDAQ in April 2021. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
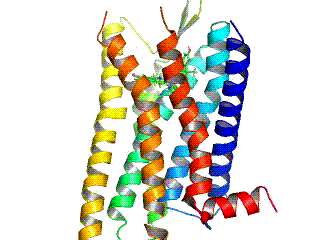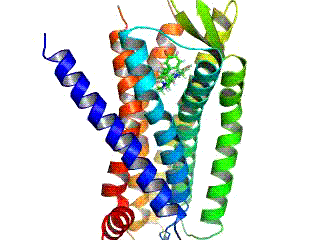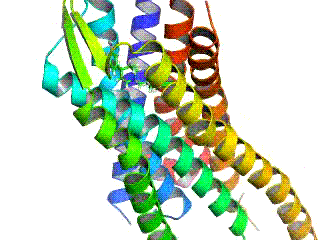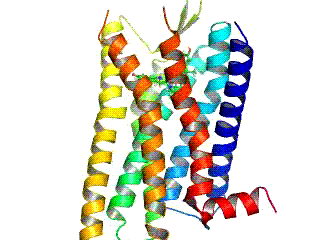
Alpha-3 Beta-4 Nicotinic Receptor
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor and the ganglion-type nicotinic receptor,Pharmacology, (Rang, Dale, Ritter & Moore, , 5th ed., Churchill Livingstone 2003) p. 138. is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the autonomic ganglia and adrenal medulla, where activation yields post- and/or presynaptic excitation, mainly by increased Na+ and K+ permeability. As with other nicotinic acetylcholine receptors, the α3β4 receptor is pentameric α3)m(β4)n where m + n = 5 The exact subunit stoichiometry is not known and it is possible that more than one functional α3β4 receptor assembles in vivo with varying subunit stoichiometries. Ligands which inhibit the α3β4 receptor have been shown to modulate drug-seeking behavior, making α3β4 a promising target for the development of novel antiaddictive agents. Ligands Agonists * Acetylcholine (endogenous neurotransmitter that binds non- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-Methoxycoronaridine
18-Methoxycoronaridine (18-MC, or MM-110) is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction. 18-MC was in the early stages of human testing by the California-based drug development company Savant HWP before being acquired by MindMed, a Canadian pharmaceutical company newly listed on the NASDAQ in April 2021. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogaine
Ibogaine is a naturally occurring psychoactive substance found in plants in the family Apocynaceae such as ''Tabernanthe iboga'', ''Voacanga africana'', and ''Tabernaemontana undulata''. It is a psychedelic with dissociative properties. Preliminary research indicates that it may help counter drug addiction. Its use has been associated with serious side effects and death. Between the years 1990 and 2008, a total of 19 fatalities temporally associated with the ingestion of ibogaine were reported, from which six subjects died of acute heart failure or cardiopulmonary arrest. The total number of subjects who have used it without major side effects during this period remains unknown. It is used as an alternative medicine treatment for drug addiction in some countries. Its prohibition in other countries has slowed scientific research. Ibogaine is also used to facilitate psychological introspection and spiritual exploration. Various derivatives of ibogaine designed to lack psychedel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Rehabilitation
Drug rehabilitation is the process of medical or psychotherapeutic treatment for dependency on psychoactive substances such as alcohol, prescription drugs, and street drugs such as cannabis, cocaine, heroin or amphetamines. The general intent is to enable the patient to confront substance dependence, if present, and stop substance misuse to avoid the psychological, legal, financial, social, and physical consequences that can be caused. Treatment includes medication for depression or other disorders, counseling by experts and sharing of experience with other addicts. Psychological dependency Psychological dependency is addressed in many drug rehabilitation programs by attempting to teach the person new methods of interacting in a drug-free environment. In particular, patients are generally encouraged, or possibly even required, to not associate with peers who still use the addictive substance. Twelve-step programs encourage addicts not only to stop using alcohol or other d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voacangine
Voacangine (12-methoxyibogamine-18-carboxylic acid methyl ester) is an alkaloid found predominantly in the root bark of the ''Voacanga africana'' tree, as well as in other plants such as ''Tabernanthe iboga'', ''Tabernaemontana africana'', ''Trachelospermum jasminoides'', ''Tabernaemontana divaricata'' and ''Ervatamia yunnanensis''. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol. Pharmacology Pharmacodynamics Voacangine exhibits AChE inhibitory activity. Docking simulation reveals that it has inhibitory effect on VEGF2 kinase and reduces angiogenesis. Like ibogaine, its a potent HERG blocker in vitro. It also acts as antagonist to TRPM8 and TRPV1 receptor but agonist of TRPA1. Pharmacokinetics The a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noribogaine
Noribogaine (actually O-desmethylibogaine), or 12-hydroxyibogamine, is the principal psychoactive metabolite of the oneirogen ibogaine. It is thought to be involved in the antiaddictive effects of ibogaine-containing plant extracts, such as ''Tabernanthe iboga''. Pharmacology Noribogaine is a potent serotonin reuptake inhibitor, but does not affect the reuptake of dopamine. Unlike ibogaine, noribogaine does not bind to the sigma-2 receptor. Similarly to ibogaine, noribogaine acts as a weak NMDA receptor antagonist and binds to opioid receptors. It has greater affinity for each of the opioid receptors than does ibogaine. Noribogaine is a hERG inhibitor and appears at least as potent as ibogaine. The inhibition of the hERG potassium channel delays the repolarization of cardiac action potentials, resulting in QT interval prolongation and, subsequently, in arrhythmias and sudden cardiac arrest. κ-Opioid receptor Noribogaine has been determined to act as a biased agonist of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogaine
Ibogaine is a naturally occurring psychoactive substance found in plants in the family Apocynaceae such as ''Tabernanthe iboga'', ''Voacanga africana'', and ''Tabernaemontana undulata''. It is a psychedelic with dissociative properties. Preliminary research indicates that it may help counter drug addiction. Its use has been associated with serious side effects and death. Between the years 1990 and 2008, a total of 19 fatalities temporally associated with the ingestion of ibogaine were reported, from which six subjects died of acute heart failure or cardiopulmonary arrest. The total number of subjects who have used it without major side effects during this period remains unknown. It is used as an alternative medicine treatment for drug addiction in some countries. Its prohibition in other countries has slowed scientific research. Ibogaine is also used to facilitate psychological introspection and spiritual exploration. Various derivatives of ibogaine designed to lack psychedel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coronaridine
Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in ''Tabernanthe iboga'' and related species, including ''Tabernaemontana divaricata'' for which (under the now obsolete synonym ''Ervatamia coronaria'') it was named. Like ibogaine, (''R'')-coronaridine and (''S'')-coronaridine can decrease intake of cocaine and morphine intake in animals and it may have muscle relaxant and hypotensive activity. Chemistry Congeners Coronaridine congers are important in drug discovery and development due to multiple actions on different targets. They have ability to inhibit Cav2.2 channel, modulate and inhibit subunits of nAChr selectively such as α9α10, α3β4 and potentiate GABAA activity. Pharmacology Coronaridine has been reported to bind to an assortment of molecular sites, including: μ-opioid (Ki = 2.0 μM), δ-opioid (Ki = 8.1 μM), and κ-opioid receptors (Ki = 4.3 μM), NMDA receptor (Ki = 6.24 μM) (as an antagonist), and nAChRs (as an antagonist). It h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid Receptor
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract. Discovery By the mid-1960s, it had become apparent from pharmacologic studies that opiate drugs were likely to exert their actions at specific receptor sites, and that there were likely to be multiple such sites. Early studies had indicated that opiates appeared to accumulate in the brain. The receptors were first identified as specific molecules through the use of binding studies, in which opiates that had been labeled with radioisotopes were found to bind to brain membrane homogenates. The first such study was published in 1971, using 3H-levorphanol. In 1973, Candace Pert and Solomon H. Snyder publis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kappa Opioid Receptor
Kappa (uppercase Κ, lowercase κ or cursive ; el, κάππα, ''káppa'') is the 10th letter of the Greek alphabet, representing the voiceless velar plosive sound in Ancient and Modern Greek. In the system of Greek numerals, has a value of 20. It was derived from the Phoenician letter kaph . Letters that arose from kappa include the Roman K and Cyrillic К. The uppercase form is identical to the Latin K. Greek proper names and placenames containing kappa are often written in English with "c" due to the Romans' transliterations into the Latin alphabet: Constantinople, Corinth, Crete. All formal modern romanizations of Greek now use the letter "k", however. The cursive form is generally a simple font variant of lower-case kappa, but it is encoded separately in Unicode for occasions where it is used as a separate symbol in math and science. In mathematics, the kappa curve is named after this letter; the tangents of this curve were first calculated by Isaac Barrow in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_with_tube_model.png)