|
13C NMR Acetonide Analysis
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundance of such stable isotopes can be measured experimentally (isotope analysis), yielding an isotope ratio that can be used as a research tool. Theoretically, such stable isotopes could include the radiogenic daughter products of radioactive decay, used in radiometric dating. However, the expression stable-isotope ratio is preferably used to refer to isotopes whose relative abundances are affected by isotope fractionation in nature. This field is termed stable isotope geochemistry. Stable-isotope ratios Measurement of the ratios of naturally occurring stable isotopes (isotope analysis) plays an important role in isotope geochemistry, but stable isotopes (mostly hydrogen, carbon, nitrogen, oxygen and sulfur) are also finding uses in ecological ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
I-food
A heavy isotope diet is one in that contains nutrients in which some atoms are replaced with their heavier non-radioactive isotopes, such as deuterium 2H or heavy carbon 13C. Biomolecules that incorporate heavier isotopes give rise to more stable molecular structures, which is hypothesized to increase resistance to damage associated with ageing or diseases. Medicines with some hydrogen atoms substituted with deuterium are called deuterated drugs, while substances that are essential nutrients can be used as food constituents, making this food "isotopic". Consumed with food, these nutrients become building material for the body. The examples are deuterated polyunsaturated fatty acids, essential aminoacids, DNA bases such as cytosine, or heavy water and glucose. Suggested mechanism One of the most pernicious and irreparable types of oxidative damage inflicted by reactive oxygen species (ROS) upon biomolecules involves the carbon-hydrogen bond cleavage (hydrogen abstraction). Int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
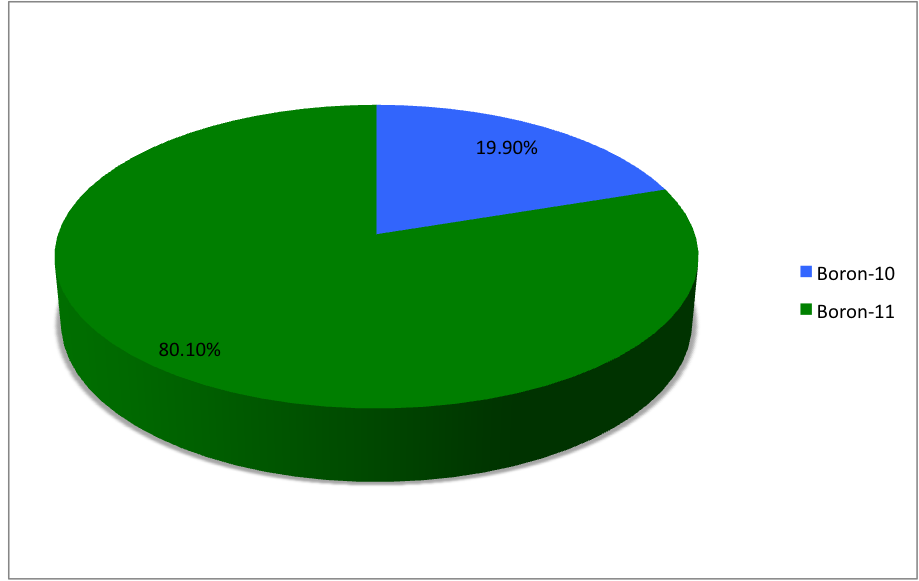
Boron-13
Boron (5B) naturally occurs as isotopes and , the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-lives, the longest being that of , with a half-life of only and with a half-life of . All other isotopes have half-lives shorter than . Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for and ) while those with mass above 11 mostly become carbon. List of isotopes , - , ?This isotope has not yet been observed; given data is inferred or estimated from periodic trends. , style="text-align:center" , 5 , style="text-align:center" , 1 , , p-unstable , 2p? , ? , 2−# , , , - , , style="text-align:center" , 5 , style="text-align:center" , 2 , , [] , p , Subsequently decays by double proton emission to for a net reaction of → + 3 , (3/2−) , , , - , Has 1 halo nucleus, halo proton , style="text- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz N. D. Kurie, Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (), which makes up 99% of all carbon on Earth; carbon-13 (), which makes up 1%; and carbon-14 (), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Carbon-14 decays into nitrogen-14 () through bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Fractionation
Isotope fractionation describes fractionation processes that affect the relative abundance of isotopes, phenomena which are taken advantage of in isotope geochemistry and other fields. Normally, the focus is on stable isotopes of the same element. Isotopic fractionation can be measured by isotope analysis, using isotope-ratio mass spectrometry or cavity ring-down spectroscopy to measure ratios of isotopes, an important tool to understand geochemical and biological systems. For example, biochemical processes cause changes in ratios of stable carbon isotopes incorporated into biomass. Definition Stable isotopes partitioning between two substances ''A'' and ''B'' can be expressed by the use of the isotopic fractionation factor (alpha): where ''R'' is the ratio of the heavy to light isotope (e.g., 2H/1H or 18O/16O). Values for alpha tend to be very close to 1. McGlynn, Shawn E.; "Biological Isotope Fractionation and Earth History: From Enzymes to Cells to Ecosystems" pp 59-79 in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Carbon
Carbon (6C) has 15 known isotopes, from to , of which and are stable nuclide, stable. The longest-lived radionuclide, radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature—trace quantities are formed cosmogenic nuclide, cosmogenically by the reaction + → + . The most stable artificial radioisotope is , which has a half-life of . All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The least stable isotope is , with a half-life of . List of isotopes , - , , style="text-align:right" , 6 , style="text-align:right" , 2 , , [] , proton emission, 2p , Subsequently decays by double proton emission to for a net reaction of → + 4 , 0+ , , , - , rowspan=3, , rowspan=3 style="text-align:right" , 6 , rowspan=3 style="text-align:right" , 3 , rowspan=3, , rowspan=3, , β+ () , , rowspan=3, 3/2− , rowspan=3, , rowspan=3, , - , β+α () , Immediately decays ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases. It has also been detected on other plane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helicobacter Pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, microaerophilic, spiral (helical) bacterium usually found in the stomach. Its helical shape (from which the genus name, helicobacter, derives) is thought to have evolved in order to penetrate the mucoid lining of the stomach and thereby establish infection. The bacterium was first identified in 1982 by the Australian doctors Barry Marshall and Robin Warren. ''H. pylori'' has been associated with cancer of the mucosa-associated lymphoid tissue in the stomach, esophagus, colon, rectum, or tissues around the eye (termed extranodal marginal zone B-cell lymphoma of the cited organ), and of lymphoid tissue in the stomach (termed diffuse large B-cell lymphoma). ''H. pylori'' infection usually has no symptoms but sometimes causes gastritis (stomach inflammation) or ulcers of the stomach or first part of the small intestine. The infection is also associated with the development of cer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13 Nuclear Magnetic Resonance
Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR ( NMR) and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the isotope. The main carbon isotope, is not detected. Although much less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds. Chemical shifts 13C NMR chemical shifts follow the same principles as those of 1H, although the typical range of chemical shifts is much larger than for 1H (by a factor of about 20). The chemical shift reference standard for 13C is the carbons in tetramethylsilane (TMS), whose chemical shift is considered to be 0.0 ppm. ImageSize = width:540 height:440 AlignBars ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permian Extinction
The Permian ( ) is a geologic period and stratigraphic system which spans 47 million years from the end of the Carboniferous Period million years ago (Mya), to the beginning of the Triassic Period 251.9 Mya. It is the last period of the Paleozoic Era; the following Triassic Period belongs to the Mesozoic Era. The concept of the Permian was introduced in 1841 by geologist Sir Roderick Murchison, who named it after the region of Perm in Russia. The Permian witnessed the diversification of the two groups of amniotes, the synapsids and the sauropsids (reptiles). The world at the time was dominated by the supercontinent Pangaea, which had formed due to the collision of Euramerica and Gondwana during the Carboniferous. Pangaea was surrounded by the superocean Panthalassa. The Carboniferous rainforest collapse left behind vast regions of desert within the continental interior. Amniotes, which could better cope with these drier conditions, rose to dominance in place of their amphibian ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stomatal Conductance
Stomatal conductance, usually measured in mmol m−2 s−1 by a porometer, estimates the rate of gas exchange (i.e., carbon dioxide uptake) and transpiration (i.e., water loss as water vapor) through the leaf stomata as determined by the degree of stomatal aperture (and therefore the physical resistances to the movement of gases between the air and the interior of the leaf). The stomatal conductance, or its inverse, stomatal resistance, is under the direct biological control of the leaf through its guard cells, which surround the stomatal pore. The turgor pressure and osmotic potential of guard cells are directly related to the stomatal conductance. Stomatal conductance is a function of stomatal density, stomatal aperture, and stomatal size. Stomatal conductance is integral to leaf level calculations of transpiration. Multiple studies have shown a direct correlation between the use of herbicides and changes in physiological and biochemical growth processes in plants, particularly n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |