|
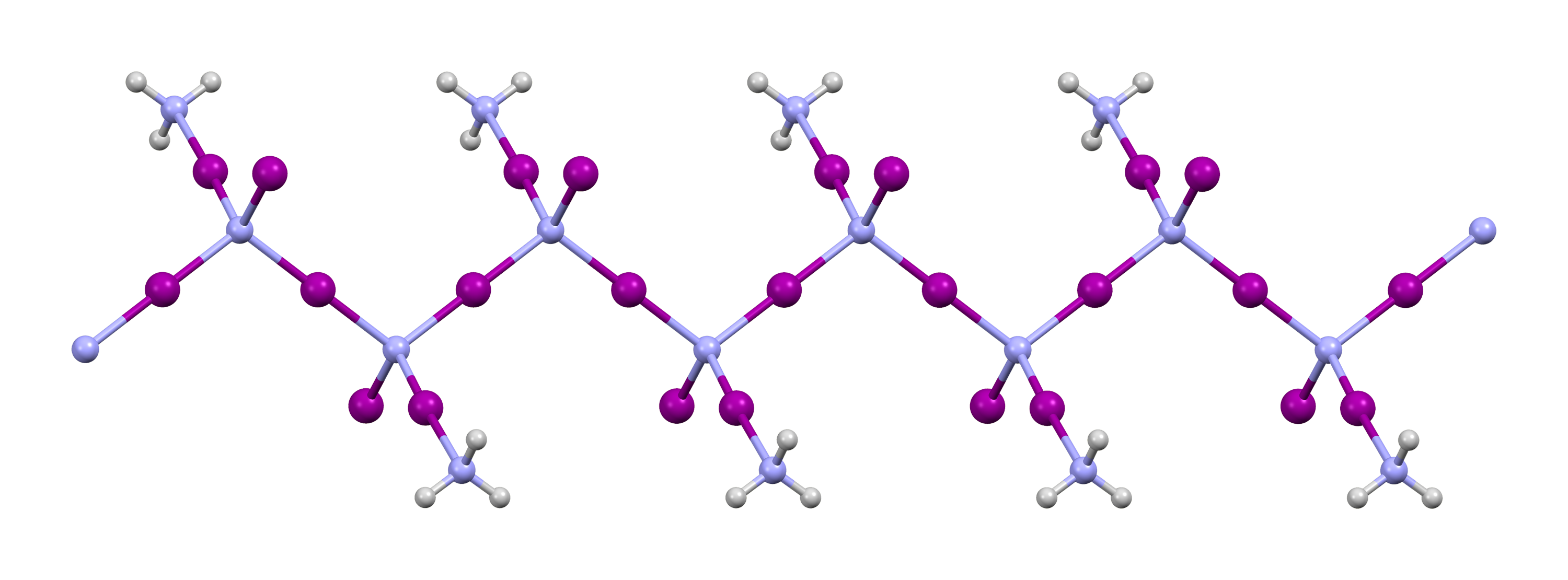
1-diazidocarbamoyl-5-azidotetrazole
1-Diazidocarbamoyl-5-azidotetrazole, often jokingly referred to as azidoazide azide, is a heterocyclic inorganic compound with the formula C2N14. It is an extremely sensitive explosive. Synthesis 1-Diazidocarbamoyl-5-azidotetrazole was produced by diazotizing triaminoguanidinium chloride with sodium nitrite in ultra-purified water. Another synthesis uses a metathesis reaction between isocyanogen tetrabromide in acetone and aqueous sodium azide. This first forms isocyanogen tetraazide, the "open" isomer of C2N14, which at room temperature quickly undergoes an irreversible cyclization reaction to form a tetrazole ring. Properties The C2N14 molecule is a monocyclic tetrazole with three azide groups with a molecular weight of 220.16 g.mol−1. It has a molecular equilibrium between a closed and an open form, isocyanogen tetraazide which has been known since 1961, the latter being quickly cyclized to the cyclic tetrazole form (C2N14) at room temperature. It is one of a family o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be * chemical energy, such as nitroglycerin or grain dust * pressurized gas, such as a gas cylinder, aerosol can, or BLEVE * nuclear energy, such as in the fissile isotopes uranium-235 and plutonium-239 Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high explosives" and materials that deflagrate are said to be "low explosives". Explosives may al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Explosives
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be * chemical energy, such as nitroglycerin or grain dust * pressurized gas, such as a gas cylinder, aerosol can, or BLEVE * nuclear energy, such as in the fissile isotopes uranium-235 and plutonium-239 Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high explosives" and materials that deflagrate are said to be "low explosives". Explosives may al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazoles
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated. Structure and bonding Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1''H''-, 2''H''-, and 5''H''-tetrazole. The 1''H''- and 2''H''- isomers are tautomers, with the equilibrium lying on the side of 1''H''-tetrazole in the solid phase. In the gas phase, 2''H''-tetrazole dominates. These isomers can be regarded as aromatic, with 6 π-electrons, while the 5''H''-isomer is nonaromatic. Synthesis 1''H''-Tetrazole was first prepared by the reaction of anhydrous hydrazoic acid and hydrogen cyanide under pressure. Treatment of organic nitriles with sodium azide in the presence of iodine or silica-supported sodium bisulfate as a heterogeneous catalyst enables an advantageous synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Triiodide
Nitrogen triiodide is an inorganic compound with the formula N I3. It is an extremely sensitive contact explosive: small quantities explode with a loud, sharp snap when touched even lightly, releasing a purple cloud of iodine vapor; it can even be detonated by alpha radiation. NI3 has a complex structural chemistry that is difficult to study because of the instability of the derivatives. Although nitrogen is more electronegative than iodine, the compound was so named due to its analogy to the compound nitrogen trichloride. Structure of NI3 and its derivatives Nitrogen triiodide was first characterized by Raman spectroscopy in 1990 when it was prepared by an ammonia-free route. Boron nitride reacts with iodine monofluoride in trichlorofluoromethane at −30 °C to produce pure NI3 in low yield: :BN + 3 IF → NI3 + BF3 NI3 is pyramidal (C3v molecular symmetry), as are the other nitrogen trihalides and ammonia. The material that is usually called "nitrogen triiodide" is pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can be described ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated. Structure and bonding Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1''H''-, 2''H''-, and 5''H''-tetrazole. The 1''H''- and 2''H''- isomers are tautomers, with the equilibrium lying on the side of 1''H''-tetrazole in the solid phase. In the gas phase, 2''H''-tetrazole dominates. These isomers can be regarded as aromatic, with 6 π-electrons, while the 5''H''-isomer is nonaromatic. Synthesis 1''H''-Tetrazole was first prepared by the reaction of anhydrous hydrazoic acid and hydrogen cyanide under pressure. Treatment of organic nitriles with sodium azide in the presence of iodine or silica-supported sodium bisulfate as a heterogeneous catalyst enables an advantageous synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |