|
1,4-Bis(diphenylphosphino)butane
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents. Coordination complexes Nickel complexes in which the ligand is bidentate or monodentate are known. Palladium complexes containing dppb are used in a variety of catalytic reactions. The ligand's natural bite angle is 94° in its bidentate coordination mode. Related compounds *1,2-Bis(dimethylphosphino)ethane *Bis(diphenylphosphino)methane *1,3-Bis(diphenylphosphino)propane 1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula PhP(CH)PPh. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It i ... References {{DEFAULTSORT:Bis(diphenylphosphino)butane, 1,4- Chelating agents Diphosphines Phenyl compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts. Synthesis 222px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl + 2 NaPPh2 → Ph2P(CH2)nPPh2 + 2 NaCl Diphosphine ligands can also be prepare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
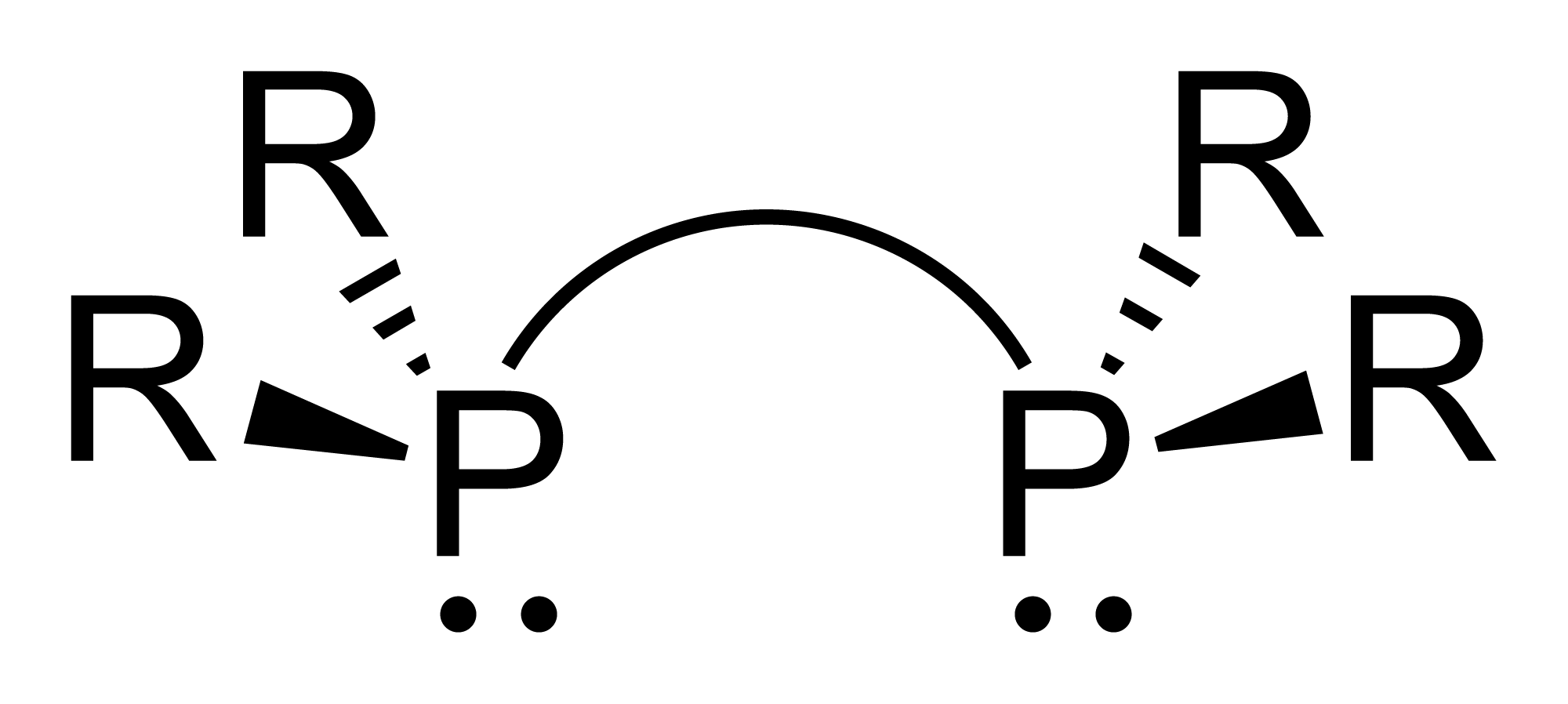
Diphosphine
Diphosphane, or diphosphine, is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air. Properties, preparation, reactions Diphosphane adopts the gauche conformation (like hydrazine, less symmetrical than shown in the image) with a P−P distance of 2.219 angstroms. It is nonbasic, unstable at room temperature, and Pyrophoricity, spontaneously flammable in air. It is only poorly soluble in water but dissolves in organic solvents. Its 1H NMR spectrum consists of 32 lines resulting from an A2XX'A'2 splitting system. Diphosphane is produced by the hydrolysis of calcium monophosphide, which can be described as the Ca2+ derivative of . According to an optimized procedure, hydrolysis of 400 g of CaP at −30 °C gives about 20 g of product, slightly contaminated with phosphine. Reaction of diphosphane with butyllithium affords a varie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(diphenylphosphino)ethane
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Preparation The preparation of dppe is by the alkylation of NaPPh: :P(CH) + 2 Na → NaP(CH) + NaCH NaP(CH), which is readily air-oxidized, is treated with 1,2-dichloroethane (ClCHCHCl) to give dppe: :2 NaP(CH) + ClCHCHCl → (CH)PCHCHP(CH) + 2 NaCl Reactions The reduction of dppe by lithium to give PhHP(CH)PHPh has been reported. :PhP(CH)PPh + 4 Li → PhLiP(CH)PLiPh + 2 PhLi Hydrolysis gives the bis(secondary phosphine): :PhLiP(CH)PLiPh + 2 PhLi + 4HO → PhHP(CH)PHPh + 4 LiOH + 2 CH : Treatment of dppe with conventional oxidants such as hydrogen peroxide (HO), aqueous bromine (Br), etc., produces dppeO in low yield (e.g., 13%) as a result of non-selective oxidation.Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd Select ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monodentate
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate (sometimes called unidentate). Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms. Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used. Bridging ligands use the μ ('mu') notation. Classes Polydentate ligands are chelating agents and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or mor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bite Angle
In coordination chemistry the bite angle is the ligand–metal–ligand bond angle of coordination complex containing a bidentate ligand. This geometric parameter is used to classify chelation, chelating ligands, including those in organometallic complexes. It is most often discussed in terms of catalysis, as changes in bite angle can affect not just the activity and selectivity of a catalytic reaction but even allow alternative reaction pathways to become accessible. Although the parameter can be applied generally to any chelating ligand, it is commonly applied to describe diphosphine ligands, as they can adopt a wide range of bite angles. Diamines Diamines form a wide range of coordination complexes. They typically form 5- and 6-membered chelate rings. Examples of the former include ethylenediamine and 2,2'-bipyridine, 2,2′-bipyridine. Six-membered chelate rings are formed by 1,3-diaminopropane. The bite angle in such complexes is usually near 90°. Longer chain diamines, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Bis(dimethylphosphino)ethane
1,2-Bis(dimethylphosphino)ethane (dmpe) is a diphosphine ligand in coordination chemistry. It is a colorless, air-sensitive liquid that is soluble in organic solvents. With the formula (CHPMe), dmpe is used as a compact strongly basic spectator ligand (Me = methyl), Representative complexes include V(dmpe)(BH), Mn(dmpe)(AlH), Tc(dmpe)(CO)Cl, and Ni(dmpe)Cl. Synthesis It is synthesised by the reaction of methylmagnesium iodide with 1,2-bis(dichlorophosphino)ethane: :ClPCHCHPCl + 4 MeMgI → MePCHCHPMe + 4 MgICl Alternatively it can be generated by alkylation of sodium dimethylphosphide. The synthesis of dmpe from thiophosphoryl chloride has led to serious accidents and has been abandoned.} Related ligands * Bis(dicyclohexylphosphino)ethane, a bulkier analogue, which is also a solid. * 1,2-Bis(diphenylphosphino)ethane, more air-stable than dmpe, but less basic. * 1,2-Bis(dimethylphosphino)benzene, a more rigid analogue of dmpe. * Tetramethylethylenediamine Tetrame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(diphenylphosphino)methane
1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°. Synthesis and reactivity 1,1-Bis(diphenylphosphino)methane was first prepared by the reaction of sodium diphenylphosphide (Ph2PNa) with dichloromethane: :Ph3P + 2 Na → Ph2PNa + NaPh :2NaPPh2 + CH2Cl2 → Ph2PCH2PPh2 + 2 NaCl The methylene group (CH2) in dppm (and especially its complexes) is mildly acidic. The ligand can be oxidized to give the corresponding oxides and sulfides CH2 (E)Ph2sub>2 (E = O, S). The methylene group is even more acidic in these derivatives. Coordination chemistry As a chelating ligand, 1,1-bis(diphenylphosphino)methane forms a four-membered ring with the constituents MP2C. The ligand promotes the format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



2.jpg)
-3D-balls.png)
2.png)
2-from-xtal-3D-ball-stick-hybrid.png)