|
1,1,1,2-Tetrachloroethane
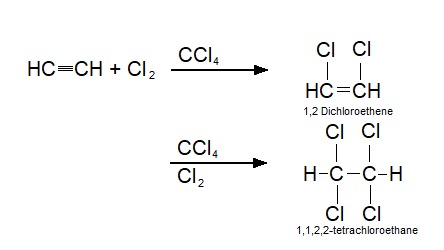
1,1,1,2-Tetrachloroethane is a chlorinated hydrocarbon. It is a colorless liquid with a sweet chloroform-like odor. It is used as a solvent and in the production of wood stains and varnishes. See also * 1,1,2,2-Tetrachloroethane 1,1,2,2-tetrachloroethane (TeCA), also known as bonoform, cellon, or westron is a toxic, synthetic halogen rich alkane. It is colorless liquid and has a sweet odor. It is used as an industrial solvent or as a separation agent. TeCA can be inhaled, c ... References Chloroalkanes Halogenated solvents IARC Group 2B carcinogens Sweet-smelling chemicals {{organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,2,2-Tetrachloroethane
1,1,2,2-tetrachloroethane (TeCA), also known as bonoform, cellon, or westron is a toxic, synthetic halogen rich alkane. It is colorless liquid and has a sweet odor. It is used as an industrial solvent or as a separation agent. TeCA can be inhaled, consumed or absorbed through the skin. After exposure, nausea, dizziness or even liver damage may occur. History 1,1,2,2-tetrachloroethane was used in large amounts to produce other chemicals like trichloroethylene, tetrachloroethylene, and 1,2,-dichloroethylene. It also found its function as a industrial solvent and was used in paint removers and pesticides. Because of its possible carcinogen effects on humans, the production of 1,1,2,2-tetrachloroethane has decreased significantly and is no longer widely used as an end-product. It is however still generated as a byproduct and as an intermediate product during manufacturing, where low levels of the chemical have been detected in the air. Synthesis There are a few different ways ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorinated Hydrocarbon
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. Aliphatic organochlorides are often alkylating agents as chlorine can act as a leaving group, which can result in cellular damage. Natural occurrence Many organochlorine compounds have been isola ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Structure The molecule adopts a tetrahedral molecular geometry with C3v symmetry group, symmetry. Natural occurrence The total global flux of chloroform through the environment is approximately tonnes per year, and about 90% of emissions are natural in origin. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. Abiotic processes are also believed to contribute to natural chloroform productions in soils although the mechanism is still unclear. Chloroform volatilizes readily from soil and surface water and undergoes degradation in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wood Stain
Wood stain is a type of paint used to colour wood and consists of colourants dissolved and/or suspended in a 'vehicle' or solvent. Vehicle is the preferred term, as the contents of a stain may not be truly dissolved in the vehicle, but rather suspended, and thus the vehicle may not be a true solvent. The vehicle often may be water, alcohol, a petroleum distillate, or a finishing agent such as shellac, lacquer, varnish and polyurethane. Coloured or stained finishes do not typically deeply penetrate the pores of the wood and may largely disappear when the finish deteriorates or is removed. Pigments and/or dyes are largely used as colourants in most stains. The difference between the two is in the solubility and in the size of the particles. While dyes are molecules that dissolve into the vehicle, pigments are larger than molecules and are temporarily suspended in the vehicle, usually settling out over time. Stains with primarily dye content are said to be 'transparent', while st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Varnish
Varnish is a clear transparent hard protective coating or film. It is not a stain. It usually has a yellowish shade from the manufacturing process and materials used, but it may also be pigmented as desired, and is sold commercially in various shades. Varnish is primarily used as a wood finish where, stained or not, the distinctive tones and grains in the wood are intended to be visible. Varnish finishes are naturally glossy, but satin/semi-gloss and flat sheens are available. History The word "varnish" comes from Mediaeval Latin ''vernix'', meaning odorous resin, itself derived from Middle Greek ''berōnikón'' or ''beroníkē'', meaning amber or amber-colored glass. A false etymology traces the word to the Greek ''Berenice'', the ancient name of modern Benghazi in Libya, where the first varnishes in the Mediterranean area were supposedly used and where resins from the trees of now-vanished forests were sold. Early varnishes were developed by mixing resin—pine sap, for ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroalkanes
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. Aliphatic organochlorides are often alkylating agents as chlorine can act as a leaving group, which can result in cellular damage. Natural occurrence Many organochlorine compounds have been isolate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogenated Solvents
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |