|
1-Naphthylamine
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer (transitional cell carcinoma). It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.. Preparation and reactions It can be prepared by reducing 1-nitronaphthalene with iron and hydrochloric acid followed by steam distillation. Oxidizing agents, such as ferric chloride, give a blue precipitate with solutions of its salts. Chromic acid converts it into 1-naphthoquinone. Sodium in boiling amyl alcohol reduces the unsubstituted ring, giving tetrahydro-1-naphthylamine. This tetrahydro compound yields adipic acid when oxidized by potassium permanganate. At 200 °C in sulfuric acid, it converts to 1-naphthol. Use in dyes The sulfonic acid derivatives of 1-naphthylamine are used for the preparation of azo dye. These compounds possess the important pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses aniline Aniline is an organic compound with the formula C6 H5 NH2. Consi .... It is an industrially significant Commodity chemicals, commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It Combustion, ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the ''precipitant''. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'supernate' or 'supernatant'. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (''e.g.'', metallurgy and alloys) when solid impurities segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The formati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthionic Acid
Naphthionic acid is an organic compound with the formula C10H6(SO3H)(NH2). It is one of several aminonaphthalenesulfonic acids, derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear gray.''4-Amino-1-naphthalenesulfonic acid;'' MSDS No. 250619; Sigma–Aldrich Chemie GmbH: Steinheim, 29 Dec 2011. It is used in the synthesis of azo dyes such as Rocceline (a. k. a. Solid Red A), during which the amino group of the acid (in the form of a salt) is diazotated and then coupled with, in the case mentioned, β-naphthol. It is prepared by treating 1-aminonaphthalene with sulfuric acid Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ....Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus ''Gossypium'' in the mallow family Malvaceae. The fiber is almost pure cellulose, and can contain minor percentages of waxes, fats, pectins, and water. Under natural conditions, the cotton bolls will increase the dispersal of the seeds. The plant is a shrub native to tropical and subtropical regions around the world, including the Americas, Africa, Egypt and India. The greatest diversity of wild cotton species is found in Mexico, followed by Australia and Africa. Cotton was independently domesticated in the Old and New Worlds. The fiber is most often spun into yarn or thread and used to make a soft, breathable, and durable textile. The use of cotton for fabric is known to date to prehistoric times; fragments of cotton fabric dated to the fifth millennium BC have been found in the Indus Valley civilization, as well as fabric remnants dated back ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
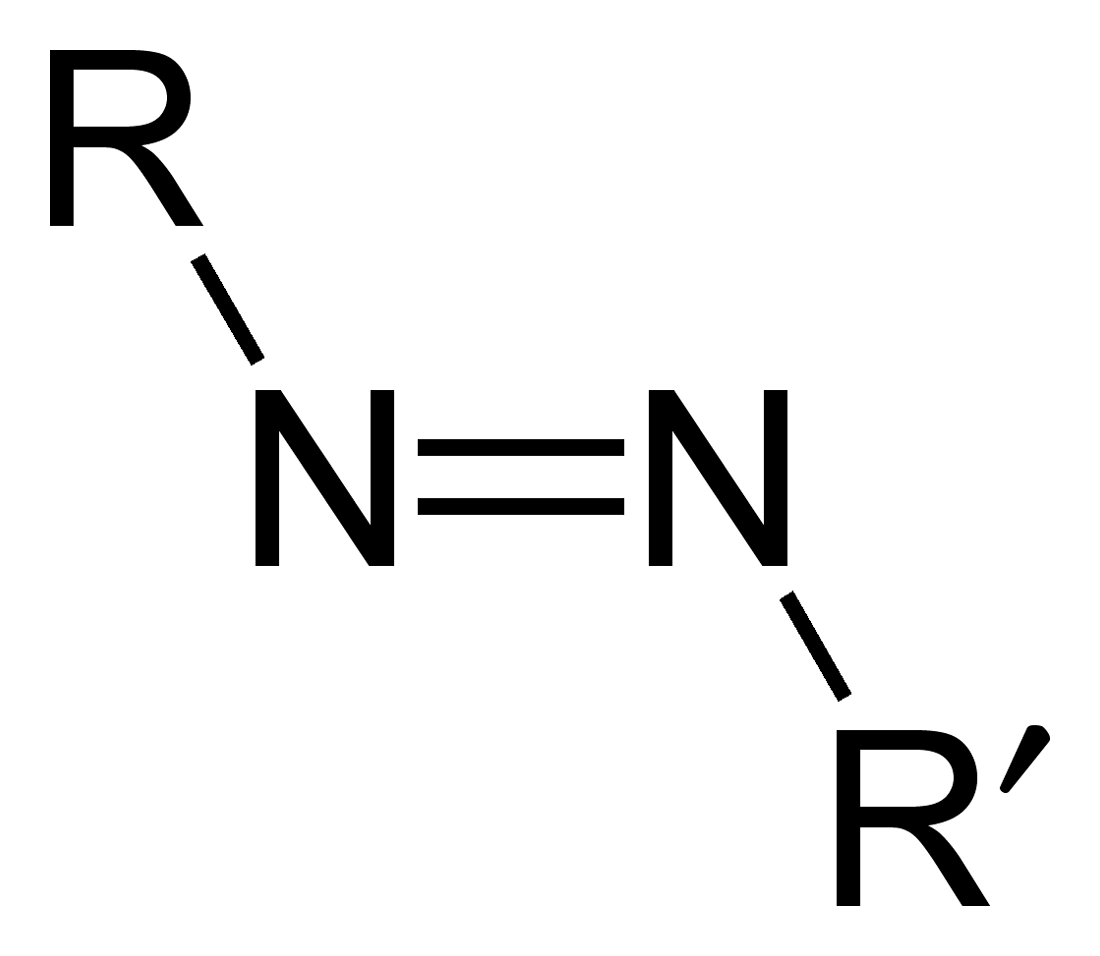
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminonaphthalenesulfonic Acids
Aminonaphthalenesulfonic acids are compounds with the composition C10H6(NH2)(SO3H), being derived from naphthalene (C10H8) substituted by an amino and sulfonic acid groups. These compounds are colorless solids. They are useful precursors to dyes.Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. . Notes: Peri-acid dehydrates to the sultam. Via the Bucherer reaction, heating periacid with anilinium salts gives the N-phenyl derivative, precursor to Acid Blue 113. {, class="wikitable" style="margin-left: auto; margin-right: auto; border: none;" , + 2-Aminonaphthalenesulfonic acids , - ! scope="col" , Isomer ! scope="col" , CAS Registry Number ! scope="col" , Alternative names ! scope="col" , Preparative route and notes , - , 2-Aminonaphthalene-1-sulfonic acid, , 81-16-3, , Tobias acid, , Bucherer reaction The Bucherer reaction in organic chemistry is the reversible conversion of a naphthol to a naphthylami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4− centre is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidize
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adipic Acid
Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Preparation and reactivity Adipic acid is produced from a mixture of cyclohexanone and cyclohexanol called KA oil, the abbreviation of ketone-alcohol oil. The KA oil is oxidized with nitric acid to give adipic acid, via a multistep pathway. Early in the reaction, the cyclohexanol is converted to the ketone, releasing nitrous acid: :HOC6H11 + HNO3 → OC(CH2)5 + HNO2 + H2O Among its many reactions, the cyclohexanone is nitrosated, setting the stage for the scission of the C-C bond: :HNO2 + HNO3 → NO+NO3− + H2O :OC6H10 + NO+ → OC6H9-2-NO + H+ Side products of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyl Alcohol
An amyl alcohol is any of eight alcohols with the formula C5H12O. A mixture of pentyl, amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol. Amyl alcohol is used as a solvent and in esterification, by which is produced amyl acetate and other important products. The name ''amyl alcohol'' without further specification applies to the normal (straight-chain) form, 1-Pentanol, 1-pentanol. These are the 8 alcohols that are structural isomers with molecular formula C5H12O: : Three of these alcohols, 2-methyl-1-butanol, 2-pentanol, and 3-methyl-2-butanol (methyl isopropyl carbinol), are therefore optical isomerism, optically active. The most important amyl alcohol is isoamyl alcohol, the chief one generated by fermentation in the production of alcoholic beverages and a constituent of fusel oil. The other amyl alcohols may be obtained synthetically. References {{DEFAULTSORT:Amyl Alcohol Alkanols GABAA receptor positive allosteric modulators ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

-3D-balls.png)