|
δ15N
In geochemistry, hydrology, paleoclimatology and paleoceanography, ''δ''15N (pronounced "delta fifteen n") or delta-N-15 is a measure of the ratio of the two stable isotopes of nitrogen, 15N: 14N. Formulas Two very similar expressions for are in wide use in hydrology. Both have the form 1000\cdot\fraca ‰ (‰ = permil or parts per thousand) where ''s'' and ''a'' are the relative abundances of 15N in respectively the sample and the atmosphere. The difference is whether the relative abundance is with respect to all the nitrogen, i.e. 14N plus 15N, or just to 14N. Since the atmosphere is 99.6337% 14N and 0.3663% 15N, ''a'' is 0.003663 in the former case and 0.003663/0.996337 = 0.003676 in the latter. However ''s'' varies similarly; for example if in the sample 15N is 0.385% and 14N is 99.615%, ''s'' is 0.003850 in the former case and 0.00385/0.99615 = 0.003865 in the latter. The value of 1000\cdot\fraca is then 51.05‰ in the former case and 51.38‰ in the latter, an in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Isotopic Signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis. Stable isotopes The atomic mass of different isotopes affect their chemical kinetic behavior, leading to natural isotope separation processes. Carbon isotopes For example, different sources and sinks of methane have different affinity for the 12C and 13C isotopes, which allows distinguishing between different sources by the 13C/12C ratio in methane in the air. In geochemistry, paleoclimatology and paleoceanography this ratio is called δ13C. The ratio is calculated with respect to Pee Dee Belemnite (PDB) standard: :\delta \ce_\mathrm = \left(\frac - 1\right) \cdot 1000 ‰ Similarly, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
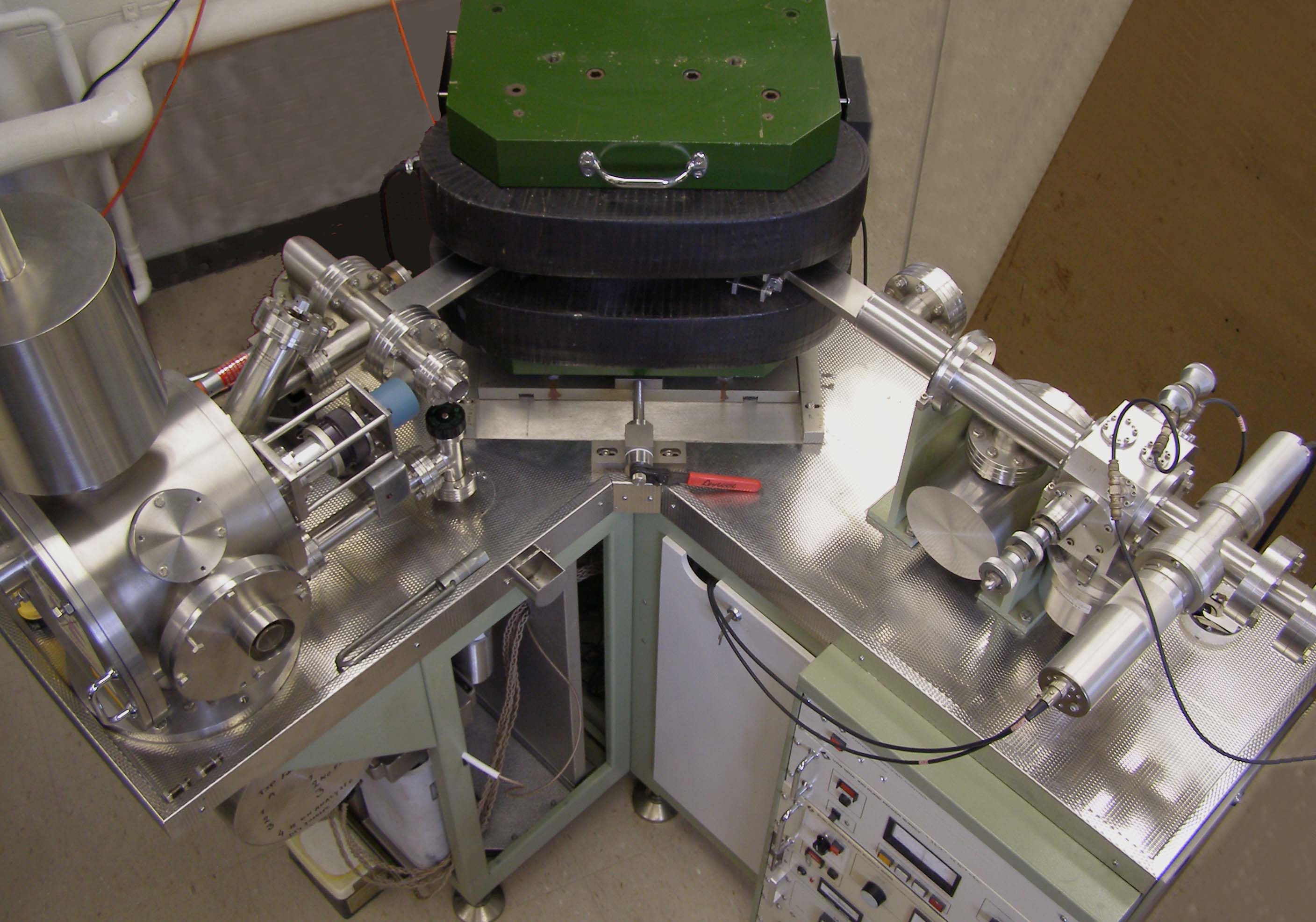
Isotope Analysis
Isotope analysis is the identification of isotopic signature, abundance of certain stable isotopes of chemical elements within organic and inorganic compounds. Isotopic analysis can be used to understand the flow of energy through a food web, to reconstruct past environmental and climatic conditions, to investigate human and animal diets, for food authentification, and a variety of other physical, geological, palaeontological and chemical processes. Stable isotope ratios are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio. Tissues affected Isotopic oxygen is incorporated into the body primarily through ingestion at which point it is used in the formation of, for archaeological purposes, bones and teeth. The oxygen is incorporated into the hydroxylcarbonic apatite of bone and tooth enamel. Bone is continually remodelled throughout the lifetime of an individual. Although the rate of tur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Geochemistry
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the entire Solar System, and has made important contributions to the understanding of a number of processes including mantle convection, the formation of planets and the origins of granite and basalt. It is an integrated field of chemistry and geology. History The term ''geochemistry'' was first used by the Swiss-German chemist Christian Friedrich Schönbein in 1838: "a comparative geochemistry ought to be launched, before geognosy can become geology, and before the mystery of the genesis of our planets and their inorganic matter may be revealed." However, for the rest of the century the more common term was "chemical geology", and there was little contact between geologists and chemists. Geochemistry emerged as a separate discipline after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |