|
β-phellandrene
Phellandrenes are organic compounds with the formula . They have a similar molecular structure and similar chemical properties. α-Phellandrene and β-phellandrene are cyclic terpene, monoterpenes and are double-bond isomers. In α-phellandrene, both double bonds are endocyclic, and in β-phellandrene, one of them is exocyclic. Both are insoluble in water, but miscibility, miscible with organic solvents. Etymology and occurrence α-Phellandrene was named after ''Eucalyptus phellandra'', now called ''Eucalyptus radiata'', from which it can be isolated. It is also a constituent of the essential oil of ''Eucalyptus dives''. β-Phellandrene has been isolated from the oil of fennel, water fennel and Canada balsam oil. The main source of β-phellandrene is terpentine. β-pinene is a source of β-phellandrene. Reactions and uses α-Phellandrene undergoes hydrochlorination to give phellandrene hydrochloride (a cyclohexenyl chloride). Base hydrolysis of this hydrochloride gives pipe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Merck Index
''The Merck Index'' is an encyclopedia of chemicals, drugs and biologicals with over 10,000 monographs on single substances or groups of related compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an appendix with monographs on organic named reactions. The 15th edition was published in A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium Trichloride
Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·''x''H2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry. Preparation and properties Anhydrous ruthenium(III) chloride is usually prepared by heating powdered ruthenium metal with chlorine. In the original synthesis, the chlorination was conducted in the presence of carbon monoxide, the product being carried by the gas stream and crystallising upon cooling. Two polymorphs of RuCl3 are known. The black α-form adopts the CrCl3-type structure with long Ru-Ru contacts of 346 pm. This polymorph has honeycomb layers of Ru3+ which are surrounded with an octahedral cage of Cl− anions. The ruthenium cations are magnetic residing in a low-spin J~1/2 ground stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated Dienes
Conjugation or conjugate may refer to: Linguistics *Grammatical conjugation, the modification of a verb from its basic form *Emotive conjugation or Russell's conjugation, the use of loaded language Mathematics *Complex conjugation, the change of sign of the imaginary part of a complex number * Conjugate (square roots), the change of sign of a square root in an expression *Conjugate element (field theory), a generalization of the preceding conjugations to roots of a polynomial of any degree *Conjugate transpose, the complex conjugate of the transpose of a matrix * Harmonic conjugate in complex analysis * Conjugate (graph theory), an alternative term for a line graph, i.e. a graph representing the edge adjacencies of another graph *In group theory, various notions are called conjugation: **Inner automorphism, a type of conjugation homomorphism **Conjugacy class In mathematics, especially group theory, two elements a and b of a group are conjugate if there is an element g in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoterpenes
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1cB-elimination reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism, Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C molecular geometry, Pi bond''). The specifics of the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a Substrate (chemistry), substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic Carbon–hydrogen bond, C−H bonds are about 15% weaker than the C−H bonds in ordinary Orbital hybridisation, sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranyl Pyrophosphate
Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many thousands of natural product, natural products. Occurrence GPP is an intermediate in the Terpenoid, isoprenoid biosynthesis pathway that produces longer prenyl chains such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate as well as many Terpene, terpenes. It can be prepared in the laboratory from geraniol. Microbial toxicity Intracellularly produced GPP has been shown to be toxic to the bacteria ''E. coli'' at moderate doses. Related compounds * Geraniol * Farnesyl pyrophosphate * Geranylgeranyl pyrophosphate See also * Dimethylallyltranstransferase References Further reading *Kulkarni RS, Pandit SS, Chidley HG, Nagel R, Schmidt A, Gershenzon J, Pujari KH, Giri AP and Gupta VS, 2013Characterization of three novel isoprenyl diphosphate synthases from the terpenoid rich mango fruit Plant Phy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN1 Reaction
The unimolecular nucleophilic substitution (SN1) reaction is a substitution reaction in organic chemistry. The Hughes-Ingold symbol of the mechanism expresses two properties—"SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is molecularity, unimolecular. Thus, the rate equation is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using Steady state (chemistry), steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with Alcohol (chemistry), secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction, SN2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
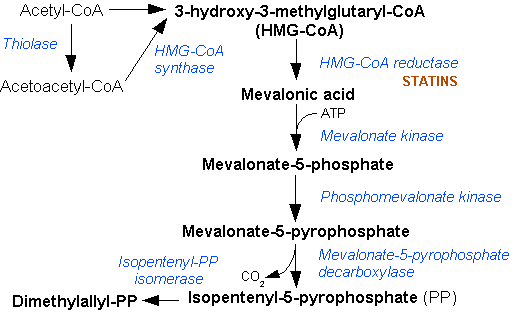
Isopentenyl Pyrophosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer Dimethylallyl pyrophosphate, DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids. Biosynthesis IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase. IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate, (''E'')-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |