|
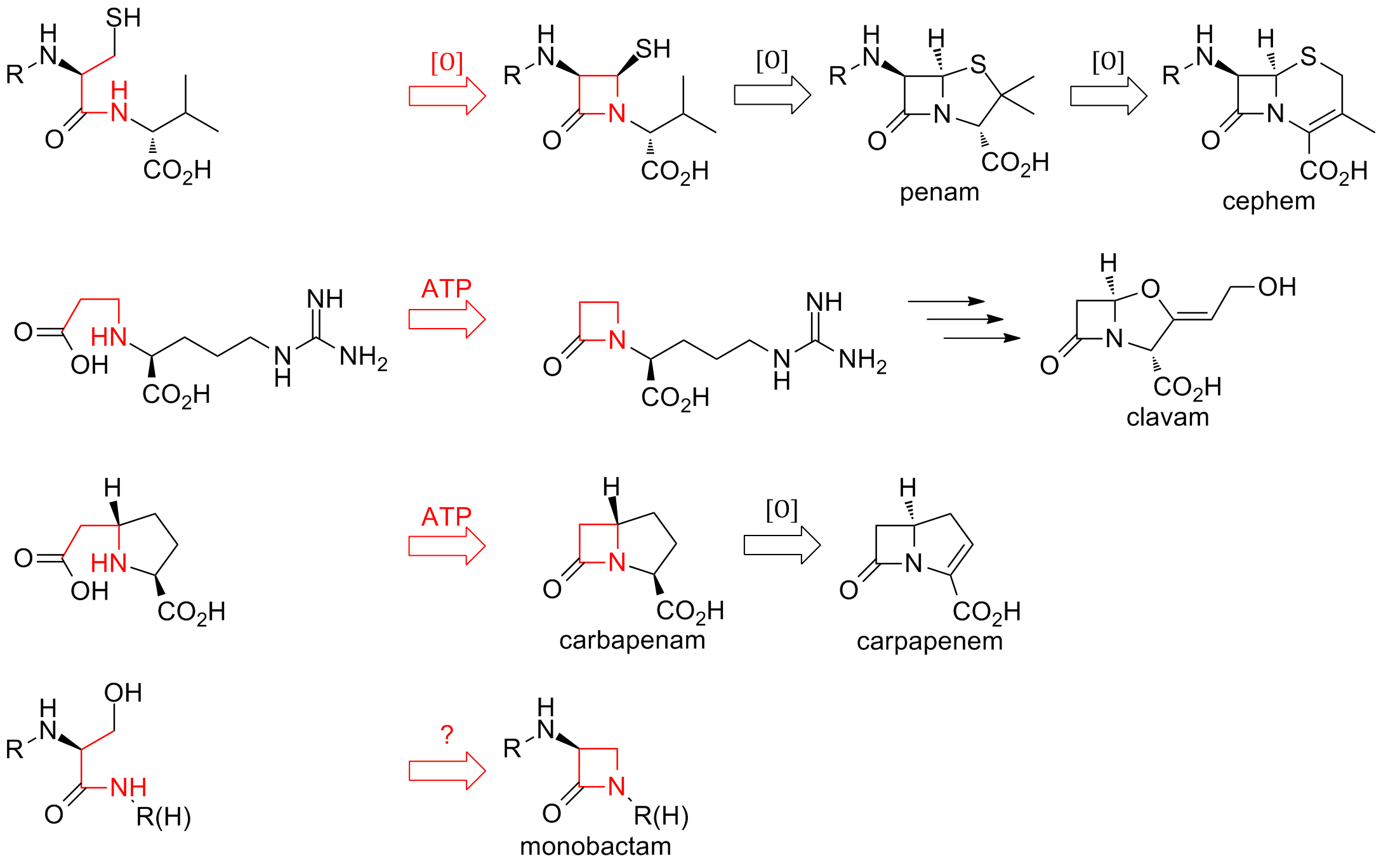
β-lactams
A beta-lactam (β-lactam) ring is a four-membered lactam. A ''lactam'' is a cyclic amide, and ''beta''-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described. Clinical significance The β-lactam ring is part of the core structure of several antibiotic families, the principal ones being the penicillins, cephalosporins, carbapenems, and monobactams, which are, therefore, also called β-lactam antibiotics. Nearly all of these antibiotics work by inhibiting bacterial cell wall biosynthesis. This has a lethal effect on bacteria, although any given bacteria population will typically contain a subgroup that is resistant to Π... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-lactam Antibiotic
β-lactam antibiotics (beta-lactam antibiotics) are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a rare variant of ''Penicillium notatum'' (since renamed ''Penicillium chrysogenum)''. Bacteria often develop resistance to β-lactam antibiotics by synthesizing a β-lactamase, an enzyme that attacks the β-lactam ring. To overcome this resistance, β-lactam antibiotics can be given with β-lactamase inhibitors such as clavulanic acid. Medical use β-lactam antibiotics are indicated for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-lactam Antibiotic
β-lactam antibiotics (beta-lactam antibiotics) are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a strain of ''Penicillium rubens'' (named as ''Penicillium notatum'' at the time). Bacteria often develop resistance to β-lactam antibiotics by synthesizing a β-lactamase, an enzyme that attacks the β-lactam ring. To overcome this resistance, β-lactam antibiotics can be given with β-lactamase inhibitors such as clavulanic acid. Medical use β-lactam antibiotics are indicated fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-lactam
A beta-lactam (β-lactam) ring is a four-membered lactam. A ''lactam'' is a cyclic amide, and ''beta''-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described. Clinical significance The β-lactam ring is part of the core structure of several antibiotic families, the principal ones being the penicillins, cephalosporins, carbapenems, and monobactams, which are, therefore, also called β-lactam antibiotics. Nearly all of these antibiotics work by inhibiting bacterial cell wall biosynthesis. This has a lethal effect on bacteria, although any given bacteria population will typically contain a subgroup that is resistant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-azetidinone
A beta-lactam (β-lactam) ring is a four-membered lactam. A ''lactam'' is a cyclic amide, and ''beta''-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described. Clinical significance The β-lactam ring is part of the core structure of several antibiotic families, the principal ones being the penicillins, cephalosporins, carbapenems, and monobactams, which are, therefore, also called β-lactam antibiotics. Nearly all of these antibiotics work by inhibiting bacterial cell wall biosynthesis. This has a lethal effect on bacteria, although any given bacteria population will typically contain a subgroup that is resistant to Π... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin Core
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. rubens''. Most penicillins in clinical use are synthesised by Penicillium chrysogenum, P. chrysogenum using industrial fermentation, deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: benzylpenicillin, penicillin G (intramuscular injection, intramuscular or Intravenous therapy, intravenous use) and phenoxymethylpenicillin, penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococcus, staphylococci and streptococcus, streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed antibiotic resis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-lactamase
Beta-lactamases, (β-lactamases) are enzymes () produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties. Beta-lactam antibiotics are typically used to target a broad spectrum of gram-positive and gram-negative bacteria. Beta-lactamases produced by gram-negative bacteria are usually secreted, especially when antibiotics are present in the environment. Structure The structure of a '' Streptomyces'' serine β-lactamase (SBLs) is given by . The alpha-beta fold ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cephalosporin
The cephalosporins (sg. ) are a class of β-lactam antibiotics originally derived from the fungus ''Acremonium'', which was previously known as ''Cephalosporium''. Together with cephamycins, they constitute a subgroup of β-lactam antibiotics called cephems. Cephalosporins were discovered in 1945, and first sold in 1964. Discovery The aerobic mold which yielded cephalosporin C was found in the sea near a sewage outfall in Su Siccu, by Cagliari harbour in Sardinia, by the Italian pharmacologist Giuseppe Brotzu in July 1945. Structure Cephalosporin contains a 6-membered dihydrothiazine ring. Substitutions at position 3 generally affect pharmacology; substitutions at position 7 affect antibacterial activity, but these cases are not always true. Medical uses Cephalosporins can be indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic. First-generation cephalosporins are active predominantly against Gram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monobactam
Monobactams are monocyclic and bacterially-produced β-lactam antibiotics. The β-lactam ring is not fused to another ring, in contrast to most other β-lactams. Monobactams are effective only against aerobic Gram-negative bacteria (e.g., ''Neisseria'', ''Pseudomonas''). Siderophore-conjugated monobactams show promise for the treatment of multi drug-resistant pathogens. Aztreonam is a commercially available monobactam antibiotic. Other examples of monobactams are tigemonam, nocardicin A, and tabtoxin. Adverse effects to monobactams can include skin rash and occasional abnormal liver functions. Monobactam antibiotics exhibit no IgE cross-reactivity reactions with penicillin but have shown some cross reactivity with cephalosporins, most notably ceftazidime Ceftazidime, sold under the brand name Fortaz among others, is a third-generation cephalosporin antibiotic useful for the treatment of a number of bacterial infections. Specifically it is used for joint infections, menin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylketene
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumule. The most important reaction of diphenyl ketene is the +2cycloaddition at C-C, C-N, C-O, and C-S multiple bonds. History Diphenyl ketene was first isolated by Hermann Staudinger in 1905 and identified as the first example of the exceptionally reactive class of ketenes with the general formula R1R2C=C=O (R1=R2=phenyl group). Preparation The first synthesis by H. Staudinger was based on 2-chlorodiphenylacetyl chloride (prepared from benzilic acid and thionyl chloride) from which two chlorine atoms are cleaved with zinc in a dehalogenation reaction: An early synthesis uses benzilmonohydrazone (from Diphenylethanedione and hydrazine hydrate), which is oxidized with mercury(II)oxide and calcium sulfate to form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin Binding Proteins
Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics (except for tabtoxinine-β-lactam, which inhibits glutamine synthetase) bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases. Diversity There are a large number of PBPs, usually several in each organism, and they are found as both membrane-bound and cytoplasmic proteins. For example, Spratt (1977) reports that six different PBPs are routinely detected in all strains of ''E. coli'' ranging in molecular weight from 40,000 to 91,000. The different PBPs occur in different numbers per cell and have varied affinities for penicillin. The PBPs are usually broadly classified into high-molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schiff Base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. The term is often synonymous with azomethine which refers specifically to secondary aldimines (i.e. where R' ≠H). A number of special naming systems exist for these compounds. For instance a Schiff base derived from an aniline, where is a phenyl or a substituted phenyl, can be called an ''anil'', while bis-compounds are often referred to as salen-type compounds. The term Schiff base is normally applied to these compounds when they are being used as ligands to form coordination complexes with metal ions. Such complexes occur naturally, for instance in corrin, but the majority of Schiff bases are artificial and are used to form many important catalysts, such as Jacobsen's catalyst. Synthesis Schiff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |