|
╬▒-Ketoglutaric Acid
╬▒-Ketoglutaric acid (2-oxoglutaric acid) is one of two ketone derivatives of glutaric acid. The term "ketoglutaric acid," when not further qualified, almost always refers to the alpha variant. ╬▓-Ketoglutaric acid varies only by the position of the ketone functional group, and is much less common. Its carboxylate, ╬▒-ketoglutarate also called 2-oxoglutarate, is an important biological compound. It is the keto acid produced by deamination of glutamate, and is an intermediate in the Krebs cycle. Functions Alanine transaminase The enzyme alanine transaminase converts ╬▒-ketoglutarate and L-alanine to L-glutamate and pyruvate, respectively, as a reversible process. Krebs cycle ╬▒-Ketoglutarate is a key intermediate in the Krebs cycle, coming after isocitrate and before succinyl CoA. Anaplerotic reactions can replenish the cycle at this juncture by synthesizing ╬▒-ketoglutarate from transamination of glutamate, or through action of glutamate dehydrogenase on glutamate. F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure RŌĆōC(=O)ŌĆōR', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group ŌĆōC(=O)ŌĆō (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate Dehydrogenase
Glutamate dehydrogenase (GLDH, GDH) is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the ╬▒-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and ╬▒-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia (high Michaelis constant K_m of about 1 mM), and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed (that is, ╬▒-ketoglutarate and ammonia to glutamate and NAD(P)+). However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination (i.e. glutamate to ╬▒-ketoglutarate and ammonia). In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction dependi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20ŌĆō30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in natureŌĆöboth terrestrially and in the outer planets of the Solar SystemŌĆöand in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B6
Vitamin B6 is one of the B vitamins, and thus an essential nutrient. The term refers to a group of six chemically similar compounds, i.e., "vitamers", which can be interconverted in biological systems. Its active form, pyridoxal 5ŌĆ▓-phosphate, serves as a coenzyme in more than 140 enzyme reactions in amino acid, glucose, and lipid metabolism. Plants synthesize pyridoxine as a means of protection from the ultraviolet-B radiation of sunlight and to participate in synthesis of chlorophyll. Animals cannot synthesize any of the various forms of the vitamin, and hence must obtain it via diet, either of plants, or of other animals. There is some absorption of the vitamin produced by intestinal bacteria, but this is not sufficient to meet needs. For adult humans, recommendations from various countries' food regulatory agencies are in the range of 1.0 to 2.0 milligrams (mg) per day. These same agencies also recognize ill effects from intakes that are too high, and so set safe upper l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
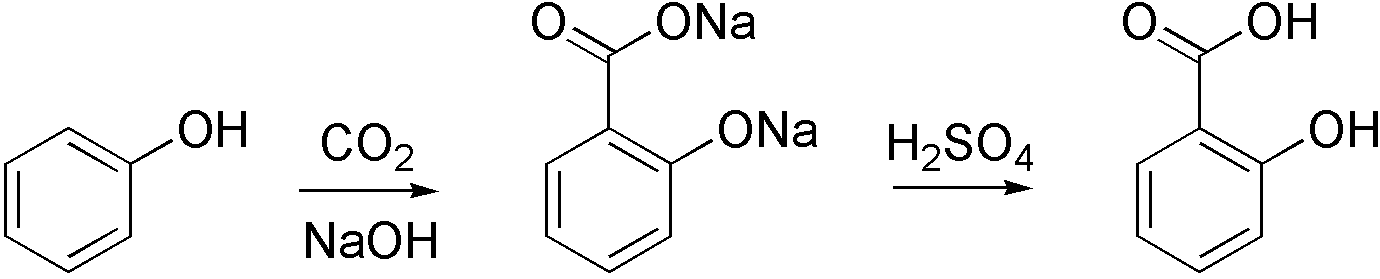
Carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) ŌĆō a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell. Neurotransmitters are released from synaptic vesicles into the synaptic cleft where they are able to interact with neurotransmitter receptors on the target cell. The neurotransmitter's effect on the target cell is determined by the receptor it binds. Many neurotransmitters are synthesized from simple and plentiful precursors such as amino acids, which are readily available and often require a small number of biosynthetic steps for conversion. Neurotransmitters are essential to the function of complex neural systems. The exact number of unique neurotransmitters in humans is unknown, but more than 100 have been identified. Common neurotransmitters include glutamate, GABA, acetylcholine, glycine and norepinephrine. Mechanism and cycle Synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic. The urea cycle converts highly toxic ammonia to urea for excretion. This cycle was the first metabolic cycle to be discovered ( Hans Krebs and Kurt Henseleit, 1932), five years before the discovery of the TCA cycle. This cycle was described in more detail later on by Ratner and Cohen. The urea cycle takes place primarily in the liver and, to a lesser extent, in the kidneys. Function Amino acid catabolism results in waste ammonia. All animals need a way to excrete this product. Most aquatic organisms, or ammonotelic organisms, excrete ammonia without converting it. Organisms that cannot easily and safely remove nitrogen as ammonia convert it to a less toxic substance, such as urea, via the urea cycle, which occurs mainly in the liver. Urea produced by the liver is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids (amino acids that can be synthesized de novo by the organism). Transamination in biochemistry is accomplished by enzymes called transaminases or aminotransferases. ╬▒-ketoglutarate acts as the predominant amino-group acceptor and produces glutamate as the new amino acid. :Aminoacid + ╬▒-ketoglutarate Ōåö ╬▒-keto acid + glutamate Glutamate's amino group, in turn, is transferred to oxaloacetate in a second transamination reaction yielding aspartate. :Glutamate + oxaloacetate Ōåö ╬▒-ketoglutarate + aspartate Mechanism of action Transamination catalyzed by aminotransferase occurs in two stages. In the first step, the ╬▒ amino group of an amino acid is transferred to the enzyme, producing the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (ŌłÆCO2ŌłÆ) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''├Īrgyros'' (ß╝äŽü╬│ŽģŽü╬┐Žé) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865ŌĆō1949) determined the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

-3D-balls.png)