Strain (chemistry) on:
[Wikipedia]
[Google]
[Amazon]
In

 The
The

 There are situations where seemingly identical conformations are not equal in strain energy. Syn-pentane strain is an example of this situation. There are two different ways to put both of the bonds the central in ''n''-pentane into a gauche conformation, one of which is 3 kcal mol−1 higher in energy than the other. When the two methyl-substituted bonds are rotated from anti to gauche in opposite directions, the molecule assumes a cyclopentane-like conformation where the two terminal methyl groups are brought into proximity. If the bonds are rotated in the same direction, this doesn't occur. The steric strain between the two terminal methyl groups accounts for the difference in energy between the two similar, yet very different conformations.
There are situations where seemingly identical conformations are not equal in strain energy. Syn-pentane strain is an example of this situation. There are two different ways to put both of the bonds the central in ''n''-pentane into a gauche conformation, one of which is 3 kcal mol−1 higher in energy than the other. When the two methyl-substituted bonds are rotated from anti to gauche in opposite directions, the molecule assumes a cyclopentane-like conformation where the two terminal methyl groups are brought into proximity. If the bonds are rotated in the same direction, this doesn't occur. The steric strain between the two terminal methyl groups accounts for the difference in energy between the two similar, yet very different conformations.
 Allylic strain, or A1,3 strain is closely associated to syn-pentane strain. An example of allylic strain can be seen in the compound 2-pentene. It's possible for the
Allylic strain, or A1,3 strain is closely associated to syn-pentane strain. An example of allylic strain can be seen in the compound 2-pentene. It's possible for the
 In principle, angle strain can occur in acyclic compounds, but the phenomenon is rare.
In principle, angle strain can occur in acyclic compounds, but the phenomenon is rare.
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
experiences strain when its chemical structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of at ...
undergoes some stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
which raises its internal energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinet ...
in comparison to a strain-free reference compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
. The internal energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinet ...
of a molecule consists of all the energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy
In physics, the elastic potential energy gained by a wire during elongation with a tensile (stretching) force is called strain energy. For linearly elastic materials, strain energy is:
: U = \frac 1 2 V \sigma \epsilon = \frac 1 2 V E \epsilon ...
, can be likened to a compressed spring
Spring(s) may refer to:
Common uses
* Spring (season)
Spring, also known as springtime, is one of the four temperate seasons, succeeding winter and preceding summer. There are various technical definitions of spring, but local usage of ...
.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors.
Common types of potential energy include the gravitational potentia ...
, a molecule can be held in an energetically unfavorable conformation by the bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released.
Summary
Thermodynamics
The equilibrium of two molecular conformations is determined by the difference inGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and ...
of the two conformations. From this energy difference, the equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for the two conformations can be determined.
:
If there is a decrease in Gibbs free energy from one state to another, this transformation is spontaneous and the lower energy state is more stable. A highly strained, higher energy molecular conformation
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of at ...
will spontaneously convert to the lower energy molecular conformation.

Enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
and entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
are related to Gibbs free energy through the equation (at a constant temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
):
:
Enthalpy is typically the more important thermodynamic function for determining a more stable molecular conformation. While there are different types of strain, the strain energy associated with all of them is due to the weakening of bonds within the molecule. Since enthalpy is usually more important, entropy can often be ignored. This isn't always the case; if the difference in enthalpy is small, entropy can have a larger effect on the equilibrium. For example, n-butane
Butane () or ''n''-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name bu ...
has two possible conformations, anti and gauche. The anti conformation is more stable by 0.9 kcal mol−1. We would expect that butane is roughly 82% anti and 18% gauche at room temperature. However, there are two possible gauche conformations and only one anti conformation. Therefore, entropy makes a contribution of 0.4 kcal in favor of the gauche conformation.Coxon and Norman, ''Principles of Organic Synthesis'', 3rd ed., Blackie Academic & Pro., 1993, We find that the actual conformational distribution of butane is 70% anti and 30% gauche at room temperature.
Determining molecular strain
 The
The standard heat of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a Chemical compound, compound is the change of enthalpy during the formation of 1 mole (unit), mole of the substance from its constituent Chemical ...
(Δf''H''°) of a compound is described as the enthalpy change when the compound is formed from its separated elements.Levine, ''Physical Chemistry'', 5th ed., McGraw-Hill, 2002, When the heat of formation for a compound is different from either a prediction or a reference compound, this difference can often be attributed to strain. For example, Δf''H''° for cyclohexane is -29.9 kcal mol−1 while Δf''H''° for methylcyclopentane
Methylcyclopentane is an organic compound with the chemical formula CH3C5 H9. It is a colourless, flammable liquid with a faint odor. It is a component of the naphthene fraction of petroleum. It usually is obtained as a mixture with cyclohexa ...
is -25.5 kcal mol−1. Despite having the same atoms and number of bonds, methylcyclopentane is higher in energy than cyclohexane. This difference in energy can be attributed to the ring strain of a five-membered ring which is absent in cyclohexane. Experimentally, strain energy is often determined using heats of combustion which is typically an easy experiment to perform.
Determining the strain energy within a molecule requires knowledge of the expected internal energy without the strain. There are two ways do this. First, one could compare to a similar compound that lacks strain, such as in the previous methylcyclohexane
Methylcyclohexane (cyclohexylmethane) is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted ...
example. Unfortunately, it can often be difficult to obtain a suitable compound. An alternative is to use Benson group increment theory Benson may refer to:
Animals
*Benson (fish), largest common carp caught in Britain
Places Geography
Canada
*Rural Municipality of Benson No. 35, Saskatchewan; rural municipality
*Benson, Saskatchewan; hamlet
United Kingdom
*Benson, Oxfordshire
...
. As long as suitable group increments are available for the atoms within a compound, a prediction of Δf''H''° can be made. If the experimental Δf''H''° differs from the predicted Δf''H''°, this difference in energy can be attributed to strain energy.
Kinds of strain
Van der Waals strain
Van der Waals strain Van der Waals strain is strain resulting from Van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their Van der Waals radii.
Van der Waals strain is also called Van der Waals repul ...
, or steric strain, occurs when atoms are forced to get closer than their Van der Waals radii allow. Specifically, Van der Waals strain is considered a form of strain where the interacting atoms are at least four bonds away from each other.Brown, Foote, and Iverson, ''Organic Chemistry'', 4th ed., Brooks/Cole, 2005, The amount on steric strain in similar molecules is dependent on the size of the interacting groups; bulky tert-butyl groups take up much more space than methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
s and often experience greater steric interactions.
The effects of steric strain in the reaction of trialkylamines and trimethylboron
Trimethylborane (TMB) is a toxic, Pyrophoricity, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl).
Properties
As a liquid it is colourless. The strongest line in the infrared spectrum is at ...
were studied by Nobel laureate Herbert C. Brown
Herbert Charles Brown (May 22, 1912 – December 19, 2004) was an American chemist and recipient of the 1979 Nobel Prize in Chemistry for his work with organoboranes.
Life and career
Brown was born Herbert Brovarnik in London, to Ukrainian Jewis ...
''et al.'' They found that as the size of the alkyl groups on the amine were increased, the equilibrium constant decreased as well. The shift in equilibrium was attributed to steric strain between the alkyl group
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
s of the amine and the methyl groups on boron.

Syn-pentane strain
Allylic strain
 Allylic strain, or A1,3 strain is closely associated to syn-pentane strain. An example of allylic strain can be seen in the compound 2-pentene. It's possible for the
Allylic strain, or A1,3 strain is closely associated to syn-pentane strain. An example of allylic strain can be seen in the compound 2-pentene. It's possible for the ethyl
Ethyl may refer to:
Arts and entertainment
* Cold Ethyl, a Swedish rock band
*Ethyl Sinclair, a character in the ''Dinosaurs'' television show
Science and technology
* Ethyl group, an organic chemistry moiety
* Ethyl alcohol (or ethanol)
* E ...
substituent of the olefin to rotate such that the terminal methyl group is brought near to the vicinal methyl group of the olefin. These types of compounds usually take a more linear conformation to avoid the steric strain between the substituents.
1,3-diaxial strain
1,3-diaxial strain is another form of strain similar to syn-pentane. In this case, the strain occurs due to steric interactions between a substituent of a cyclohexane ring ('α') and gauche interactions between the alpha substituent and both methylene carbons two bonds away from the substituent in question (hence, 1,3-diaxial interactions). When the substituent is axial, it is brought near to an axial gamma hydrogen. The amount of strain is largely dependent on the size of the substituent and can be relieved by forming into the major chair conformation placing the substituent in an equatorial position. The difference in energy between conformations is called theA value 400px, The A-value for a methyl group is 1.74 as derived from the chemical equilibrium above. This means it costs of energy to have a methyl group in the axial position compared to the equatorial position.
A-values are numerical values used in ...
and is well known for many different substituents. The A value 400px, The A-value for a methyl group is 1.74 as derived from the chemical equilibrium above. This means it costs of energy to have a methyl group in the axial position compared to the equatorial position.
A-values are numerical values used in ...
is a thermodynamic parameter and was originally measured along with other methods using the Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and ...
equation and, for example, the Meerwein–Ponndorf–Verley reduction
The Meerwein–Ponndorf–Verley (MPV) reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminium alkoxide catalysis in the presence of a sacrificial alcohol. The advantages of th ...
/Oppenauer oxidation
Oppenauer oxidation, named after , is a gentle method for selectively oxidizing secondary alcohols to ketones.
The reaction is the opposite Meerwein–Ponndorf–Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess ...
equilibrium for the measurement of axial versus equatorial values of cyclohexanone/cyclohexanol (0.7 kcal mol−1).
Torsional strain
Torsional strain is the resistance to bond twisting. In cyclic molecules, it is also called Pitzer strain. Torsional strain occurs when atoms separated by three bonds are placed in an eclipsed conformation instead of the more stable staggered conformation. The barrier of rotation between staggered conformations ofethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petroc ...
is approximately 2.9 kcal mol−1. It was initially believed that the barrier to rotation was due to steric interactions between vicinal hydrogens, but the Van der Waals radius of hydrogen is too small for this to be the case. Recent research has shown that the staggered conformation may be more stable due to a hyperconjugative effect. Rotation away from the staggered conformation interrupts this stabilizing force.
More complex molecules, such as butane, have more than one possible staggered conformation. The anti conformation of butane is approximately 0.9 kcal mol−1 (3.8 kJ mol−1) more stable than the gauche conformation. Both of these staggered conformations are much more stable than the eclipsed conformations. Instead of a hyperconjugative effect, such as that in ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petroc ...
, the strain energy in butane is due to both steric interactions between methyl groups and angle strain caused by these interactions.
Ring strain
According to theVSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm the ...
of molecular bonding, the preferred geometry of a molecule is that in which both bonding and non-bonding electrons are as far apart as possible. In molecules, it is quite common for these angles to be somewhat compressed or expanded compared to their optimal value. This strain is referred to as angle strain, or Baeyer strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane, which are discussed below. Furthermore, there is often eclipsing or Pitzer Pitzer is a surname, and may refer to:
*Alexander White Pitzer (1834–1927), American Presbyterian clergyman
*Kenneth Sanborn Pitzer (1914–1997), American theoretical chemist
*Russell Kelly Pitzer (1878–1978), American businessman and philanth ...
strain in cyclic systems. These and possible transannular interactions were summarized early by H.C. Brown as internal strain, or I-Strain. Molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
or force field approaches allow to calculate such strain contributions, which then can be correlated e.g. with reaction rates or equilibria. Many reactions of alicyclic
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached.
The ...
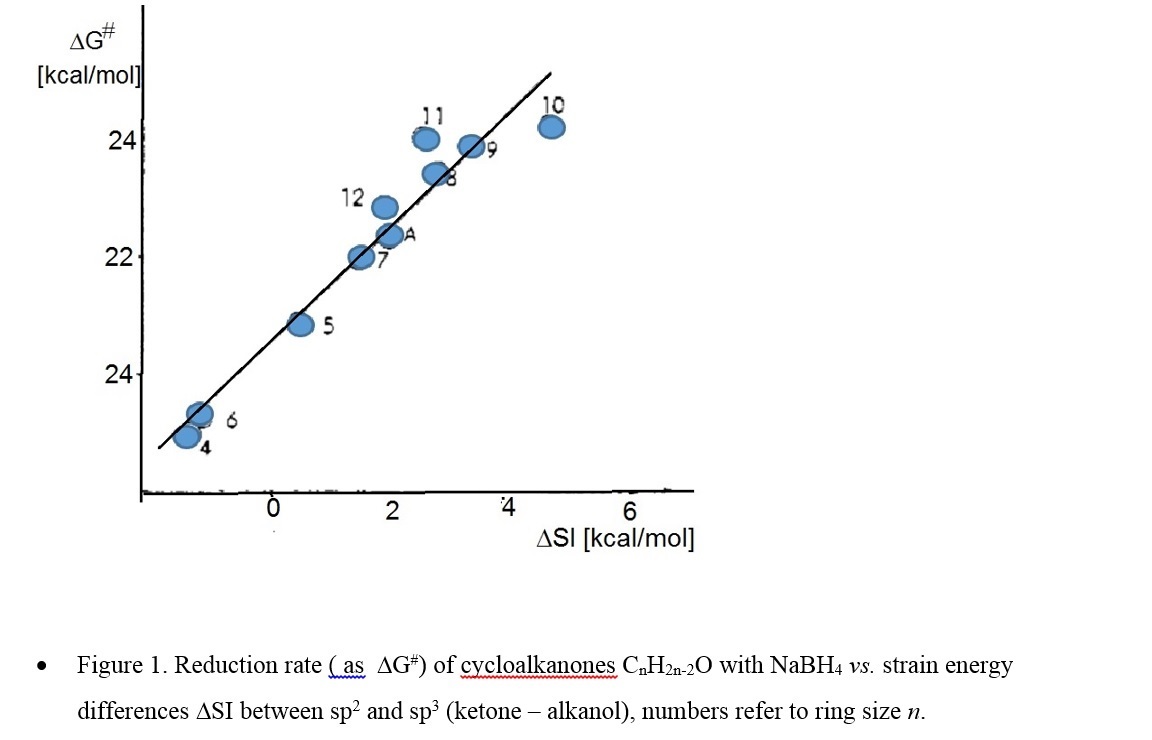
compounds, including equilibria, redox and solvolysis reactions, which all are characterized by transition between sp2 and sp3 state at the reaction center, correlate with corresponding strain energy differences SI (sp2 -sp3). The data reflect mainly the unfavourable vicinal angles in medium rings, as illustrated by the severe increase of ketone reduction rates with increasing SI (Figure 1). Another example is the solvolysis of bridgehead tosylates with steric energy differences between corresponding bromide derivatives (sp3) and the carbenium ion as sp2- model for the transition state. (Figure 2)

 In principle, angle strain can occur in acyclic compounds, but the phenomenon is rare.
In principle, angle strain can occur in acyclic compounds, but the phenomenon is rare.
Small rings
Cyclohexane is considered a benchmark in determining ring strain in cycloalkanes and it is commonly accepted that there is little to no strain energy. In comparison, smaller cycloalkanes are much higher in energy due to increased strain.Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself ...
is analogous to a triangle and thus has bond angles of 60°, much lower than the preferred 109.5° of an sp3 hybridized carbon. Furthermore, the hydrogens in cyclopropane are eclipsed. Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercia ...
experiences similar strain, with bond angles of approximately 88° (it isn't completely planar) and eclipsed hydrogens. The strain energy of cyclopropane and cyclobutane are 27.5 and 26.3 kcal mol−1, respectively. Cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occu ...
experiences much less strain, mainly due to torsional strain from eclipsed hydrogens: its preferred conformations interconvert by a process called pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chem ...
.
Ring strain can be considerably higher in bicyclic systems. For example, bicyclobutane
Bicyclobutane is an organic compound with the formula C4H6. It is a bicyclic molecule consisting of two ''cis''-fused cyclopropane rings, and is a colorless and easily condensed gas. Bicyclobutane is noted for being one of the most strained co ...
, C4H6, is noted for being one of the most strained compounds that is isolatable on a large scale; its strain energy is estimated at 63.9 kcal mol−1 (267 kJ mol−1).
Transannular strain
Medium-sized rings (7–13 carbons) experience more strain energy than cyclohexane, due mostly to deviation from ideal vicinal angles, or Pitzer strain. Molecular mechanics calculations indicate that transannular strain, also known asPrelog strain
In organic chemistry, transannular strain (also called Prelog strain after chemist Vladimir Prelog) is the unfavorable interactions of ring substituents on non-adjacent carbons. These interactions, called transannular interactions, arise from ...
, does not play an essential role. Transannular reactions however, such as 1,5-shifts in cyclooctane substitution reactions, are well known.
Bicyclic systems
The amount of strain energy in bicyclic systems is commonly the sum of the strain energy in each individual ring. This isn't always the case, as sometimes the fusion of rings induces some extra strain.Strain in allosteric systems
In synthetic allosteric systems there are typically two or more conformers with stability differences due to strain contributions. Positive cooperativity for example results from increased binding of a substrate A to a conformer C2 which is produced by binding of an effector molecule E. If the conformer C2 has a similar stability as another equilibrating conformer C1 a fit induced by the substrate A will lead to binding of A to C2 also in absence of the effector E. Only if the stability of the conformer C2 is significantly smaller, meaning that in absence of an effector E the population of C2 is much smaller than that of C1, the ratio K2/K1 which measures the efficiency of the allosteric signal will increase. The ratio K2/K1 can be related directly to the strain energy difference between the conformers C1 and C2; if it is small higher concentrations of A will directly bind to C2 and make the effector E inefficient. In addition, the response time of such allosteric switches depends on the strain of the conformer interconversion transitions state.H.-J. Schneider. ''Org. Biomol. Chem.'' 2016,14, 7994. https://pubs.rsc.org/en/content/articlepdf/2016/ob/c6ob01303aSee also
* Strain (materials science)References
{{DEFAULTSORT:Strain (Chemistry) Stereochemistry