Soil Nutrients on:
[Wikipedia]
[Google]
[Amazon]
Seventeen elements or
 Plants obtain their carbon from atmospheric carbon dioxide through
Plants obtain their carbon from atmospheric carbon dioxide through
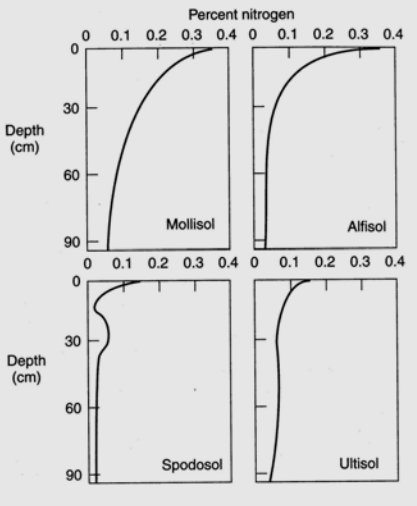 Nitrogen is the most critical element obtained by plants from the soil, to the exception of moist tropical forests where phosphorus is the limiting soil nutrient, and
Nitrogen is the most critical element obtained by plants from the soil, to the exception of moist tropical forests where phosphorus is the limiting soil nutrient, and
nutrients
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excret ...
are essential for plant growth and reproduction. They are carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
(C), hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
(H), oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
(O), nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
(N), phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
(P), potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosphe ...
(K), sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
(S), calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
(Ca), magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
(Mg), iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
(Fe), boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
(B), manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
(Mn), copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
(Cu), zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
(Zn), molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
(Mo), nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
(Ni) and chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
(Cl). Nutrients required for plants to complete their life cycle are considered essential nutrients
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excre ...
. Nutrients that enhance the growth of plants but are not necessary to complete the plant's life cycle are considered non-essential. With the exception of carbon, hydrogen and oxygen, which are supplied by carbon dioxide and water, and nitrogen, provided through nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
, the nutrients derive originally from the mineral component of the soil. The Law of the Minimum
Liebig's law of the minimum, often simply called Liebig's law or the law of the minimum, is a principle developed in agricultural science by Carl Sprengel (1840) and later popularized by Justus von Liebig. It states that growth is dictated not b ...
expresses that when the available form of a nutrient is not in enough proportion in the soil solution, then other nutrients cannot be taken up at an optimum rate by a plant. A particular nutrient ratio of the soil solution is thus mandatory for optimizing plant growth, a value which might differ from nutrient ratios calculated from plant composition.
Plant uptake of nutrients can only proceed when they are present in a plant-available form. In most situations, nutrients are absorbed in an ionic form from (or together with) soil water. Although minerals are the origin of most nutrients, and the bulk of most nutrient elements in the soil is held in crystalline form within primary and secondary minerals, they weather
Weather is the state of the atmosphere, describing for example the degree to which it is hot or cold, wet or dry, calm or stormy, clear or cloudy. On Earth, most weather phenomena occur in the lowest layer of the planet's atmosphere, the ...
too slowly to support rapid plant growth. For example, the application of finely ground minerals, feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldsp ...
and apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common e ...
, to soil seldom provides the necessary amounts of potassium and phosphorus at a rate sufficient for good plant growth, as most of the nutrients remain bound in the crystals of those minerals.
The nutrients adsorbed onto the surfaces of clay colloids and soil organic matter Soil organic matter (SOM) is the organic matter component of soil, consisting of plant and animal detritus at various stages of decomposition, cells and tissues of soil microbes, and substances that soil microbes synthesize. SOM provides numerous b ...
provide a more accessible reservoir of many plant nutrients (e.g. K, Ca, Mg, P, Zn). As plants absorb the nutrients from the soil water, the soluble pool is replenished from the surface-bound pool. The decomposition of soil organic matter Soil organic matter (SOM) is the organic matter component of soil, consisting of plant and animal detritus at various stages of decomposition, cells and tissues of soil microbes, and substances that soil microbes synthesize. SOM provides numerous b ...
by microorganisms is another mechanism whereby the soluble pool of nutrients is replenished – this is important for the supply of plant-available N, S, P, and B from soil.
Gram for gram, the capacity of humus
In classical soil science, humus is the dark organic matter in soil that is formed by the decomposition of plant and animal matter. It is a kind of soil organic matter. It is rich in nutrients and retains moisture in the soil. Humus is the Lati ...
to hold nutrients and water is far greater than that of clay minerals, most of the soil cation exchange capacity
Cation-exchange capacity (CEC) is a measure of how many cations can be retained on soil particle surfaces. Negative charges on the surfaces of soil particles bind positively-charged atoms or molecules (cations), but allow these to exchange with ot ...
arising from charged carboxylic
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
groups on organic matter. However, despite the great capacity of humus to retain water once water-soaked, its high hydrophobicity
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
decreases its wettability
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with ...
. All in all, small amounts of humus may remarkably increase the soil's capacity to promote plant growth.
Uptake processes
Nutrients in the soil are taken up by the plant through its roots, and in particular its root hairs. To be taken up by a plant, a nutrient element must be located near the root surface; however, the supply of nutrients in contact with the root is rapidly depleted within a distance of ca. 2 mm. There are three basic mechanisms whereby nutrient ions dissolved in the soil solution are brought into contact with plant roots: #Mass flow
Mass flow, also known as mass transfer and bulk flow, is the movement of fluids down a pressure or temperature gradient,Moyes & Schulte (2008). Principles of Animal Physiology. Pearson Benjamin Cummings. San Francisco, California particularly in ...
of water
# Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
within water
# Interception by root growth
All three mechanisms operate simultaneously, but one mechanism or another may be most important for a particular nutrient. For example, in the case of calcium, which is generally plentiful in the soil solution, except when aluminium over competes calcium on cation exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
sites in very acid soils (pH less than 4), mass flow alone can usually bring sufficient amounts to the root surface. However, in the case of phosphorus, diffusion is needed to supplement mass flow. For the most part, nutrient ions must travel some distance in the soil solution to reach the root surface. This movement can take place by mass flow, as when dissolved nutrients are carried along with the soil water flowing toward a root that is actively drawing water from the soil. In this type of movement, the nutrient ions are somewhat analogous to leaves floating down a stream. In addition, nutrient ions continually move by diffusion from areas of greater concentration toward the nutrient-depleted areas of lower concentration around the root surface. That process is due to random motion, also called Brownian motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas).
This pattern of motion typically consists of random fluctuations in a particle's position insi ...
, of molecules within a gradient of decreasing concentration. By this means, plants can continue to take up nutrients even at night, when water is only slowly absorbed into the roots as transpiration
Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. Water is necessary for plants but only a small amount of water taken up by the roots is used for growth a ...
has almost stopped following stoma
In botany, a stoma (from Greek ''στόμα'', "mouth", plural "stomata"), also called a stomate (plural "stomates"), is a pore found in the epidermis of leaves, stems, and other organs, that controls the rate of gas exchange. The pore is bor ...
tal closure. Finally, root interception comes into play as roots continually grow into new, undepleted soil. By this way roots are also able to absorb nanomaterials
*
Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale).
Nanomaterials research takes a materials science-based approach to nan ...
such as nanoparticulate
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
organic matter.
In the above table, phosphorus and potassium nutrients move more by diffusion than they do by mass flow in the soil water solution, as they are rapidly taken up by the roots creating a concentration of almost zero near the roots (the plants cannot transpire enough water to draw more of those nutrients near the roots). The very steep concentration gradient is of greater influence in the movement of those ions than is the movement of those by mass flow. The movement by mass flow requires the transpiration of water from the plant causing water and solution ions to also move toward the roots. Movement by root interception is slowest as the plants must extend their roots.
Plants move ions out of their roots in an effort to move nutrients in from the soil, an exchange process which occurs in the root apoplast
Inside a plant, the apoplast can mean the space outside of cell membranes, where material can diffuse freely; that is, the extracellular spaces.
''Apoplast '' can also refer especially to the continuum of cell walls of adjacent cells; fluid and ma ...
. Hydrogen H+ is exchanged for other cations, and carbonate (HCO3−) and hydroxide (OH−) anions are exchanged for nutrient anions. As plant roots remove nutrients from the soil water solution, they are replenished as other ions move off of clay and humus (by ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
or desorption
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.
There ...
), are added from the weathering
Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs ''in situ'' (on site, with little or no movement), ...
of soil minerals, and are released by the decomposition of soil organic matter. However, the rate at which plant roots remove nutrients may not cope with the rate at which they are replenished in the soil solution, stemming in nutrient limitation to plant growth. Plants derive a large proportion of their anion nutrients from decomposing organic matter, which typically holds about 95 percent of the soil nitrogen, 5 to 60 percent of the soil phosphorus and about 80 percent of the soil sulfur. Where crops are produced, the replenishment of nutrients in the soil must usually be augmented by the addition of fertilizer or organic matter.
Because nutrient uptake is an active metabolic process, conditions that inhibit root metabolism may also inhibit nutrient uptake. Examples of such conditions include waterlogging or soil compaction
In geotechnical engineering, soil compaction is the process in which stress applied to a soil causes densification as air is displaced from the pores between the soil grains. When stress is applied that causes densification due to water (or other ...
resulting in poor soil aeration, excessively high or low soil temperatures, and above-ground conditions that result in low translocation of sugars to plant roots.
Carbon
 Plants obtain their carbon from atmospheric carbon dioxide through
Plants obtain their carbon from atmospheric carbon dioxide through photosynthetic
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in c ...
carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylatio ...
, to which must be added the uptake of dissolved carbon from the soil solution and carbon transfer through mycorrhizal networks
A Mycorrhizal network (also known as a common mycorrhizal network or CMN) is an underground network found in forests and other plant communities, created by the hyphae of mycorrhizal fungi joining with plant roots. This network connects individu ...
. About 45% of a plant's dry mass is carbon; plant residues typically have a carbon to nitrogen ratio (C/N) of between 13:1 and 100:1. As the soil organic material is digested by micro-organisms
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
and saprophagous
Saprotrophic nutrition or lysotrophic nutrition is a process of chemoheterotrophic extracellular digestion involved in the processing of decayed (dead or waste) organic matter. It occurs in saprotrophs, and is most often associated with fungi ( ...
soil fauna
Soil biology is the study of microbial and faunal activity and ecology in soil.
Soil life, soil biota, soil fauna, or edaphon is a collective term that encompasses all organisms that spend a significant portion of their life cycle within a soil ...
, the C/N decreases as the carbonaceous material is metabolized and carbon dioxide (CO2) is released as a byproduct which then finds its way out of the soil and into the atmosphere. Nitrogen turnover (mostly involved in protein turnover
In cell biology, protein turnover refers to the replacement of older proteins as they are broken down within the cell. Different types of proteins have very different turnover rates.
A balance between protein synthesis and protein degradation is ...
) is lesser than that of carbon (mostly involved in respiration
Respiration may refer to:
Biology
* Cellular respiration, the process in which nutrients are converted into useful energy in a cell
** Anaerobic respiration, cellular respiration without oxygen
** Maintenance respiration, the amount of cellul ...
) in the living, then dead matter of decomposers
Decomposers are organisms that break down dead or decaying organisms; they carry out decomposition, a process possible by only certain kingdoms, such as fungi. Like herbivores and predators, decomposers are heterotrophic, meaning that they use ...
, which are always richer in nitrogen than plant litter
Plant litter (also leaf litter, tree litter, soil litter, litterfall or duff) is dead plant material (such as leaves, bark, needles, twigs, and cladodes) that have fallen to the ground. This detritus or dead organic material and its constituent ...
, and so it builds up in the soil. Normal CO2 concentration in the atmosphere is 0.03%, this can be the factor limiting plant growth. In a field of maize on a still day during high light conditions in the growing season, the CO2 concentration drops very low, but under such conditions the crop could use up to 20 times the normal concentration. The respiration of CO2 by soil micro-organisms decomposing soil organic matter and the CO2 respired by roots contribute an important amount of CO2 to the photosynthesising plants, to which must be added the CO2 respired by aboveground plant tissues. Root-respired CO2 can be accumulated overnight within hollow stems of plants, to be further used for photosynthesis during the day. Within the soil, CO2 concentration is 10 to 100 times that of atmospheric levels but may rise to toxic levels if the soil porosity is low or if diffusion is impeded by flooding.
Nitrogen
 Nitrogen is the most critical element obtained by plants from the soil, to the exception of moist tropical forests where phosphorus is the limiting soil nutrient, and
Nitrogen is the most critical element obtained by plants from the soil, to the exception of moist tropical forests where phosphorus is the limiting soil nutrient, and nitrogen deficiency
Nitrogen deficiency is a deficiency of nitrogen in plants. This can occur when organic matter with high carbon content, such as sawdust, is added to soil. Soil organisms use any nitrogen available to break down carbon sources, making nitrogen una ...
often limits plant growth. Plants can use the nitrogen as either the ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
cation (NH4+) or the anion nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
(NO3−). Plants are commonly classified as ammonium or nitrate plants according to their preferential nitrogen nutrition. Usually, most of the nitrogen in soil is bound within organic compounds that make up the soil organic matter, and must be mineralized to the ammonium or nitrate form before it can be taken up by most plants. However, symbiosis with mycorrhizal fungi
A mycorrhiza (from Greek μύκης ', "fungus", and ῥίζα ', "root"; pl. mycorrhizae, mycorrhiza or mycorrhizas) is a symbiotic association between a fungus and a plant. The term mycorrhiza refers to the role of the fungus in the plant ...
allow plants to get access to the organic nitrogen pool where and when mineral forms of nitrogen are poorly available. The total nitrogen content depends largely on the soil organic matter content, which in turn depends on texture, climate, vegetation, topography, age and soil management
Soil management is the application of operations, practices, and treatments to protect soil and enhance its performance (such as soil fertility or soil mechanics). It includes soil conservation, soil amendment, and optimal soil health. In agricult ...
. Soil nitrogen typically decreases by 0.2 to 0.3% for every temperature increase by 10 °C. Usually, grassland soils contain more soil nitrogen than forest soils, because of a higher turnover rate of grassland organic matter. Cultivation decreases soil nitrogen by exposing soil organic matter to decomposition by microorganisms, most losses being caused by denitrification
Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitr ...
, and soils under no-tillage maintain more soil nitrogen than tilled soils.
Some micro-organisms
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
are able to metabolise organic matter and release ammonium in a process called mineralisation. Others, called nitrifiers, take free ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
or nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
as an intermediary step in the process of nitrification
''Nitrification'' is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate occurring through separate organisms or direct ammonia oxidation to nitrate in comammox bacteria. The transformation of amm ...
, and oxidise it to nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
. Nitrogen-fixing bacteria
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
are capable of metabolising N2 into the form of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
or related nitrogenous compounds in a process called nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
. Both ammonium and nitrate can be immobilized by their incorporation into microbial living cells, where it is temporarily sequestered in the form of amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
and proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
. Nitrate may be lost from the soil to the atmosphere when bacteria metabolise it to the gases NH3, N2 and N2O, a process called denitrification
Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitr ...
. Nitrogen may also be leached from the vadose zone
The vadose zone, also termed the unsaturated zone, is the part of Earth between the land surface and the top of the phreatic zone, the position at which the groundwater (the water in the soil's pores) is at atmospheric pressure ("vadose" is fr ...
if in the form of nitrate, acting as a pollutant
A pollutant or novel entity is a substance or energy introduced into the environment that has undesired effects, or adversely affects the usefulness of a resource. These can be both naturally forming (i.e. minerals or extracted compounds like oi ...
if it reaches the water table
The water table is the upper surface of the zone of saturation. The zone of saturation is where the pores and fractures of the ground are saturated with water. It can also be simply explained as the depth below which the ground is saturated.
T ...
or flows over land, more especially in agricultural soils under high use of nutrient fertilizers. Ammonium may also be sequestered in 2:1 clay minerals
Clay minerals are hydrous aluminium phyllosilicates (e.g. kaolin, Al2 Si2 O5( OH)4), sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces.
Clay mineral ...
. A small amount of nitrogen is added to soil by rainfall
Rain is water droplets that have condensed from atmospheric water vapor and then fall under gravity. Rain is a major component of the water cycle and is responsible for depositing most of the fresh water on the Earth. It provides water f ...
, to the exception of wide areas of North America and West Europe where the excess use of nitrogen fertilizers
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
and manure
Manure is organic matter that is used as organic fertilizer in agriculture. Most manure consists of animal feces; other sources include compost and green manure. Manures contribute to the fertility of soil by adding organic matter and nutri ...
has caused atmospheric pollution
Air pollution is the contamination of air due to the presence of substances in the atmosphere that are harmful to the health of humans and other living beings, or cause damage to the climate or to materials. There are many different types ...
by ammonia emission, stemming in soil acidification
Soil acidification is the buildup of hydrogen cations, which reduces the soil pH. Chemically, this happens when a proton donor gets added to the soil. The donor can be an acid, such as nitric acid, sulfuric acid, or carbonic acid. It can also be a ...
and eutrophication
Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients, particularly nitrogen and phosphorus. It has also been defined as "nutrient-induced increase in phytopla ...
of soils and aquatic ecosystems
An aquatic ecosystem is an ecosystem formed by surrounding a body of water, in contrast to land-based terrestrial ecosystems. Aquatic ecosystems contain communities of organisms that are dependent on each other and on their environment. The tw ...
.
Gains
In the process of mineralisation, microbes feed on organic matter, releasing ammonia (NH3), ammonium (NH4+), nitrate (NO3−) and other nutrients. As long as the carbon to nitrogen ratio (C/N) of fresh residues in the soil is above 30:1, nitrogen will be in short supply for the nitrogen-rich microbal biomass (nitrogen deficiency
Nitrogen deficiency is a deficiency of nitrogen in plants. This can occur when organic matter with high carbon content, such as sawdust, is added to soil. Soil organisms use any nitrogen available to break down carbon sources, making nitrogen una ...
), and other bacteria will uptake ammonium and to a lesser extent nitrate and incorporate them into their cells in the immobilization process. In that form the nitrogen is said to be ''immobilised''. Later, when such bacteria die, they too are ''mineralised'' and some of the nitrogen is released as ammonium and nitrate. Predation of bacteria by soil fauna, in particular protozoa
Protozoa (singular: protozoan or protozoon; alternative plural: protozoans) are a group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic tissues and debris. Histo ...
and nematodes
The nematodes ( or grc-gre, Νηματώδη; la, Nematoda) or roundworms constitute the phylum Nematoda (also called Nemathelminthes), with plant-parasitic nematodes also known as eelworms. They are a diverse animal phylum inhabiting a broa ...
, play a decisive role in the return of immobilized nitrogen to mineral forms. If the C/N of fresh residues is less than 15, mineral nitrogen is freed to the soil and directly available to plants. Bacteria may on average add nitrogen per acre, and in an unfertilised field, this is the most important source of usable nitrogen. In a soil with 5% organic matter perhaps 2 to 5% of that is released to the soil by such decomposition. It occurs fastest in warm, moist, well aerated soil. The mineralisation of 3% of the organic material of a soil that is 4% organic matter overall, would release of nitrogen as ammonium per acre.
In nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
, rhizobium
''Rhizobium'' is a genus of Gram-negative soil bacteria that fix nitrogen. ''Rhizobium'' species form an endosymbiotic nitrogen-fixing association with roots of (primarily) legumes and other flowering plants.
The bacteria colonize plant cells ...
bacteria convert N2 to ammonia (NH3), which is rapidly converted to amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
, parts of which are used by the rhizobia for the synthesis of their own biomass proteins, while other parts are transported to the xylem
Xylem is one of the two types of transport tissue in vascular plants, the other being phloem. The basic function of xylem is to transport water from roots to stems and leaves, but it also transports nutrients. The word ''xylem'' is derived from ...
of the host plant. Rhizobia
Rhizobia are diazotrophic bacteria that fix nitrogen after becoming established inside the root nodules of legumes (Fabaceae). To express genes for nitrogen fixation, rhizobia require a plant host; they cannot independently fix nitrogen. In gene ...
share a symbiotic relationship
Symbiosis (from Greek , , "living together", from , , "together", and , bíōsis, "living") is any type of a close and long-term biological interaction between two different biological organisms, be it mutualistic, commensalistic, or parasit ...
with host plants, since rhizobia supply the host with nitrogen and the host provides rhizobia with other nutrients and a safe environment. It is estimated that such symbiotic bacteria in the root nodule
Root nodules are found on the roots of plants, primarily legumes, that form a symbiosis with nitrogen-fixing bacteria. Under nitrogen-limiting conditions, capable plants form a symbiotic relationship with a host-specific strain of bacteria known a ...
s of legume
A legume () is a plant in the family Fabaceae (or Leguminosae), or the fruit or seed of such a plant. When used as a dry grain, the seed is also called a pulse. Legumes are grown agriculturally, primarily for human consumption, for livestock f ...
s add 45 to 250 pounds of nitrogen per acre per year, which may be sufficient for the crop. Other, free-living nitrogen-fixing diazotroph Diazotrophs are bacteria and archaea that fix gaseous nitrogen in the atmosphere into a more usable form such as ammonia.
A diazotroph is a microorganism that is able to grow without external sources of fixed nitrogen. Examples of organisms that ...
bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
and archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
live independently in the soil and release mineral forms of nitrogen when their dead bodies are converted by way of mineralization.
Some amount of usable nitrogen is fixed by lightning
Lightning is a naturally occurring electrostatic discharge during which two electric charge, electrically charged regions, both in the atmosphere or with one on the land, ground, temporarily neutralize themselves, causing the instantaneous ...
as nitric oxide (NO) and nitrogen dioxide (NO2−). Nitrogen dioxide is soluble in water to form nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
(HNO3) dissociating in H+ and NO3−. Ammonia, NH3, previously emitted from the soil, may fall with precipitation as nitric acid at a rate of about five pounds nitrogen per acre per year.
Sequestration
When bacteria feed on soluble forms of nitrogen (ammonium and nitrate), they temporarily sequester that nitrogen in their bodies in a process called immobilization. At a later time when those bacteria die, their nitrogen may be released as ammonium by the process of mineralization, sped up by predatory fauna. Protein material is easily broken down, but the rate of its decomposition is slowed by its attachment to the crystalline structure of clay and when trapped between the clay layers or attached to rough clay surfaces. The layers are small enough that bacteria cannot enter. Some organisms can exude extracellular enzymes that can act on the sequestered proteins. However, those enzymes too may be trapped on the clay crystals, resulting in a complex interaction between proteins, microbial enzymes and mineral surfaces. Ammonium fixation occurs mainly between the layers of 2:1 type clay minerals such asillite
Illite is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina ...
, vermiculite
Vermiculite is a hydrous phyllosilicate mineral which undergoes significant expansion when heated. Exfoliation occurs when the mineral is heated sufficiently, and commercial furnaces can routinely produce this effect. Vermiculite forms by the wea ...
or montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite gro ...
, together with ions of similar ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation ...
and low hydration energy
In chemistry, hydration energy (also hydration enthalpy) is the amount of energy released when one mole of ions undergoes hydration. Hydration energy is one component in the quantitative analysis of solvation. It is a particular special case of ...
such as potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosphe ...
, but a small proportion of ammonium is also fixed in the silt
Silt is granular material of a size between sand and clay and composed mostly of broken grains of quartz. Silt may occur as a soil (often mixed with sand or clay) or as sediment mixed in suspension with water. Silt usually has a floury feel when ...
fraction. Only a small fraction of soil nitrogen is held this way.
Losses
Usable nitrogen may be lost from soils when it is in the form ofnitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
, as it is easily leached, contrary to ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
which is easily fixed. Further losses of nitrogen occur by denitrification
Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitr ...
, the process whereby soil bacteria convert nitrate (NO3−) to nitrogen gas, N2 or N2O. This occurs when poor soil aeration limits free oxygen, forcing bacteria to use the oxygen in nitrate for their respiratory process. Denitrification increases when oxidisable organic material is available, as in organic farming
Organic farming, also known as ecological farming or biological farming,Labelling, article 30 o''Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and re ...
and when soils are warm and slightly acidic, as currently happens in tropical areas. Denitrification may vary throughout a soil as the aeration varies from place to place. Denitrification may cause the loss of 10 to 20 percent of the available nitrates within a day and when conditions are favourable to that process, losses of up to 60 percent of nitrate applied as fertiliser may occur.
Ammonia volatilisation occurs when ammonium reacts chemically with an alkaline soil
Alkali, or Alkaline, soils are clay soils with high pH (greater than 8.5), a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico- ...
, converting NH4+ to NH3. The application of ammonium fertiliser to such a field can result in volatilisation losses of as much as 30 percent.
All kinds of nitrogen losses, whether by leaching or volatilization, are responsible for a large part of aquifer
An aquifer is an underground layer of water-bearing, permeable rock, rock fractures, or unconsolidated materials (gravel, sand, or silt). Groundwater from aquifers can be extracted using a water well. Aquifers vary greatly in their characterist ...
pollution and air pollution
Air pollution is the contamination of air due to the presence of substances in the atmosphere that are harmful to the health of humans and other living beings, or cause damage to the climate or to materials. There are many different types ...
, with concomitant effects on soil acidification
Soil acidification is the buildup of hydrogen cations, which reduces the soil pH. Chemically, this happens when a proton donor gets added to the soil. The donor can be an acid, such as nitric acid, sulfuric acid, or carbonic acid. It can also be a ...
and eutrophication
Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients, particularly nitrogen and phosphorus. It has also been defined as "nutrient-induced increase in phytopla ...
, a novel combination of environmental threats (acidity and excess nitrogen) to which extant organisms are badly adapted, causing severe biodiversity losses in natural ecosystems.
Phosphorus
After nitrogen, phosphorus is probably the element most likely to be deficient in soils, although it often turns to be the most deficient in tropical soils where the mineral pool is depleted under intenseleaching
Leaching is the loss or extraction of certain materials from a carrier into a liquid (usually, but not always a solvent). and may refer to:
* Leaching (agriculture), the loss of water-soluble plant nutrients from the soil; or applying a small amou ...
and mineral weathering
Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs ''in situ'' (on site, with little or no movement), ...
while, contrary to nitrogen, phosphorus reserves cannot be replenished from other sources. The soil mineral apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common e ...
is the most common mineral source of phosphorus, from which it can be extracted by microbial and root exudates, with an important contribution of arbuscular mycorrhizal
An arbuscular mycorrhiza (AM) (plural ''mycorrhizae'', a.k.a. ''endomycorrhiza'') is a type of mycorrhiza in which the symbiont fungus (''AM fungi'', or AMF) penetrates the cortical cells of the roots of a vascular plant forming arbuscules. ( ...
fungi. The most common form of organic phosphate is phytate
Phytic acid is a six-fold dihydrogenphosphate ester of inositol (specifically, of the ''myo'' isomer), also called inositol hexakisphosphate (IP6) or inositol polyphosphate. At physiological pH, the phosphates are partially ionized, resulting in ...
, the principal storage form of phosphorus in many plant tissues. While there is on average 1000 lb per acre (1120 kg per hectare) of phosphorus in the soil, it is generally in the form of orthophosphate
A phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. ...
with low solubility, except when linked to ammonium or calcium, hence the use of diammonium phosphate
Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid.
Solid diammonium phosp ...
or monocalcium phosphate
Monocalcium phosphate is an inorganic compound with the chemical formula Ca(H2PO4)2 ("AMCP" or "CMP-A" for anhydrous monocalcium phosphate). It is commonly found as the monohydrate ("MCP" or "MCP-M"), Ca(H2PO4)2·H2O. Both salts are colourless sol ...
as fertilizers. Total phosphorus is about 0.1 percent by weight of the soil, but only one percent of that is directly available to plants. Of the part available, more than half comes from the mineralisation of organic matter. Agricultural fields may need to be fertilised to make up for the phosphorus that has been removed in the crop.
When phosphorus does form solubilised ions of H2PO4−, if not taken up by plant roots they rapidly form insoluble phosphates of calcium or hydrous oxides of iron and aluminum. Phosphorus is largely immobile in the soil and is not leached but actually builds up in the surface layer if not cropped. The application of soluble fertilisers to soils may result in zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
deficiencies as zinc phosphate
Zinc phosphate is an inorganic compound with the formula Zn3( PO4)2. This white powder is widely used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment (see also red lea ...
s form, but soil pH levels, partly depending on the form of phosphorus in the fertiliser, strongly interact with this effect, in some cases resulting in increased zinc availability. Lack of phosphorus may interfere with the normal opening of the plant leaf stomata
In botany, a stoma (from Greek ''στόμα'', "mouth", plural "stomata"), also called a stomate (plural "stomates"), is a pore found in the epidermis of leaves, stems, and other organs, that controls the rate of gas exchange. The pore is bor ...
, decreased stomatal conductance Stomatal conductance, usually measured in mmol m−2 s−1 by a porometer, estimates the rate of gas exchange (i.e., carbon dioxide uptake) and transpiration (i.e., water loss as water vapor) through the leaf stomata as determined by the degree of ...
resulting in decreased photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
and respiration rates while decreased transpiration
Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. Water is necessary for plants but only a small amount of water taken up by the roots is used for growth a ...
increases plant temperature. Phosphorus is most available when soil pH is 6.5 in mineral soils and 5.5 in organic soils.
Potassium
The amount of potassium in a soil may be as much as 80,000 lb per acre-foot, of which only 150 lb is available for plant growth. Common mineral sources of potassium are the micabiotite
Biotite is a common group of phyllosilicate minerals within the mica group, with the approximate chemical formula . It is primarily a solid-solution series between the iron-endmember annite, and the magnesium-endmember phlogopite; more alumino ...
and potassium feldspar Potassium feldspar refers to a number of minerals in the feldspar group, and containing potassium:
*Orthoclase (endmember formula K Al Si3 O8), an important tectosilicate mineral that forms igneous rock
*Microcline, chemically the same as orthoclas ...
, KAlSi3O8. Rhizosphere
The rhizosphere is the narrow region of soil or substrate that is directly influenced by root secretions and associated soil microorganisms known as the root microbiome. Soil pores in the rhizosphere can contain many bacteria and other microor ...
bacteria, also called rhizobacteria
Rhizobacteria are root-associated bacteria that can have a detrimental (parasitic varieties), neutral or beneficial effect on plant growth. The name comes from the Greek ''rhiza'', meaning root. The term usually refers to bacteria that form symbio ...
, contribute through the production of organic acids
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are r ...
to its solubilization. When solubilised, half will be held as exchangeable cations on clay while the other half is in the soil water solution. Potassium fixation often occurs when soils dry and the potassium is bonded between layers of 2:1 expansive clay
Expansive clay is a clay soil that is prone to large volume changes (swelling and shrinking) that are directly related to changes in water content. Soils with a high content of expansive minerals can form deep cracks in drier seasons or years; su ...
minerals such as illite
Illite is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina ...
, vermiculite
Vermiculite is a hydrous phyllosilicate mineral which undergoes significant expansion when heated. Exfoliation occurs when the mineral is heated sufficiently, and commercial furnaces can routinely produce this effect. Vermiculite forms by the wea ...
or montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite gro ...
. Under certain conditions, dependent on the soil texture, intensity of drying, and initial amount of exchangeable potassium, the fixed percentage may be as much as 90 percent within ten minutes. Potassium may be leached from soils low in clay.
Calcium
Calcium is one percent by weight of soils and is generally available but may be low as it is soluble and can be leached. It is thus low in sandy and heavily leached soil or strongly acidic mineral soils, resulting in excessive concentration of free hydrogen ions in the soil solution, and therefore these soils require liming. Calcium is supplied to the plant in the form of exchangeable ions and moderately soluble minerals. There are four forms of calcium in the soil. Soil calcium can be in insoluble forms such ascalcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
or dolomite Dolomite may refer to:
*Dolomite (mineral), a carbonate mineral
*Dolomite (rock), also known as dolostone, a sedimentary carbonate rock
*Dolomite, Alabama, United States, an unincorporated community
*Dolomite, California, United States, an unincor ...
, in the soil solution in the form of a divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
or retained in exchangeable form at the surface of mineral particles. Another form is when calcium complexes with organic matter, forming covalent bonds
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
between organic compounds
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
which contribute to structural stability
In mathematics, structural stability is a fundamental property of a dynamical system which means that the qualitative behavior of the trajectories is unaffected by small perturbations (to be exact ''C''1-small perturbations).
Examples of such q ...
. Calcium is more available on the soil colloids than is potassium because the common mineral calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
, CaCO3, is more soluble than potassium-bearing minerals such as feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldsp ...
.
Calcium uptake by roots is essential for plant nutrition
Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element i ...
, contrary to an old tenet that it was luxury consumption. Calcium is considered as an essential component of plant cell membranes, a counterion
160px, Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.">cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typical ...
for inorganic and organic anions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
in the vacuole
A vacuole () is a membrane-bound organelle which is present in plant and fungal cells and some protist, animal, and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water containing inorganic and organic mo ...
, and an intracellular messenger in the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
, playing a role in cellular ''learning'' and ''memory''.
Magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
is one of the dominant exchangeable cations in most soils (after calcium and potassium). Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
is an essential element for plants, microbes and animals, being involved in many catalytic reactions and in the synthesis of chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
. Primary minerals that weather to release magnesium include hornblende
Hornblende is a complex inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common in igneous and metamorphic rocks ...
, biotite
Biotite is a common group of phyllosilicate minerals within the mica group, with the approximate chemical formula . It is primarily a solid-solution series between the iron-endmember annite, and the magnesium-endmember phlogopite; more alumino ...
and vermiculite
Vermiculite is a hydrous phyllosilicate mineral which undergoes significant expansion when heated. Exfoliation occurs when the mineral is heated sufficiently, and commercial furnaces can routinely produce this effect. Vermiculite forms by the wea ...
. Soil magnesium concentrations are generally sufficient for optimal plant growth, but highly weathered and sandy soils may be magnesium deficient due to leaching by heavy precipitation.
Sulfur
Most sulfur is made available to plants, like phosphorus, by its release from decomposing organic matter. Deficiencies may exist in some soils (especially sandy soils) and if cropped, sulfur needs to be added. The application of large quantities of nitrogen to fields that have marginal amounts of sulfur may cause sulfur deficiency by a ''dilution effect'' when stimulation of plant growth by nitrogen increases the plant demand for sulfur. A 15-ton crop of onions uses up to 19 lb of sulfur and 4 tons of alfalfa uses 15 lb per acre. Sulfur abundance varies with depth. In a sample of soils in Ohio, United States, the sulfur abundance varied with depths, 0–6 inches, 6–12 inches, 12–18 inches, 18–24 inches in the amounts: 1056, 830, 686, 528 lb per acre respectively.Micronutrients
Themicronutrients
Micronutrients are nutrient, essential dietary elements required by organisms in varying quantities throughout life to orchestrate a range of physiological functions to maintain health. Micronutrient requirements differ between organisms; for exam ...
essential in plant life, in their order of importance, include iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
, manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
, chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
and molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
. The term refers to plants' needs, not to their abundance in soil. They are required in very small amounts but are essential to plant health Plant health includes the protection of plants, as well as scientific and regulatory frameworks for controlling plant pests or pathogens. Plant health is concerned with:
*Ecosystem health with a special focus on plants
*Tree health
*The control of ...
in that most are required parts of enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
systems which are involved in plant metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
. They are generally available in the mineral component of the soil, but the heavy application of phosphates can cause a deficiency in zinc and iron by the formation of insoluble zinc and iron phosphates. Iron deficiency, stemming in plant chlorosis
In botany, chlorosis is a condition in which leaves produce insufficient chlorophyll. As chlorophyll is responsible for the green color of leaves, chlorotic leaves are pale, yellow, or yellow-white. The affected plant has little or no ability to ...
and rhizosphere
The rhizosphere is the narrow region of soil or substrate that is directly influenced by root secretions and associated soil microorganisms known as the root microbiome. Soil pores in the rhizosphere can contain many bacteria and other microor ...
acidification, may also result from excessive amounts of heavy metals or calcium minerals (lime) in the soil. Excess amounts of soluble boron, molybdenum and chloride are toxic.
Non-essential nutrients
Nutrients which enhance the health but whose deficiency does not stop the life cycle of plants include:cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
, strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
, vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
. As their importance is evaluated they may be added to the list of essential plant nutrients, as is the case for silicon.
See also
*Alkali soil
Alkali, or Alkaline, soils are clay soils with high pH (greater than 8.5), a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico ...
* Sodic soils
Soil salinity is the salt content in the soil; the process of increasing the salt content is known as salinization. Salts occur naturally within soils and water. Salination can be caused by natural processes such as mineral weathering or by the ...
* Cation-exchange capacity
Cation-exchange capacity (CEC) is a measure of how many cations can be retained on soil particle surfaces. Negative charges on the surfaces of soil particles bind positively-charged atoms or molecules (cations), but allow these to exchange with ot ...
* Soil contamination
Soil contamination, soil pollution, or land pollution as a part of land degradation is caused by the presence of xenobiotic (human-made) chemicals or other alteration in the natural soil environment. It is typically caused by industrial activity ...
* Soil fertility
Soil fertility refers to the ability of soil to sustain agricultural plant growth, i.e. to provide plant habitat and result in sustained and consistent yields of high quality.
* Index of soil-related articles
This is an index of articles relating to soil.
{{Compact ToC , side = yes , num = yes , seealso = yes , nobreak = yes , extlinks = no , center = yes
A
Acid sulfate soil
- Acrisol
- Active layer
- Agricultural soil science
- Akadama
- Albe ...
References
Bibliography
* * * ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** {{refend Soil Plant nutrition