Sultam on:
[Wikipedia]
[Google]
[Amazon]
 In
In
File:Saccharin.svg, Saccharin, a cyclic sulfonamide that was one of the first artificial sweeteners discovered
File:Sulfanilamide-skeletal.svg,
summary
{{Authority control Functional groups
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the sulfonamide functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
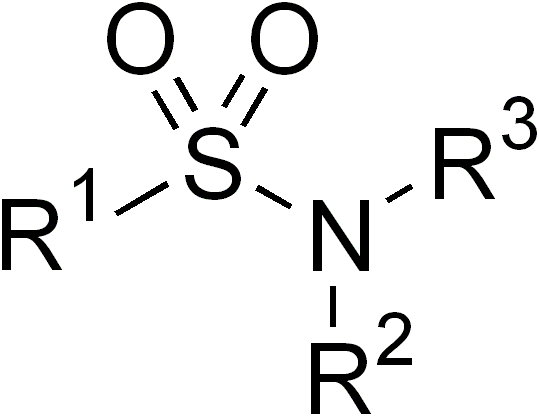
(also spelled sulphonamide) is an organosulfur group with the structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
. It consists of a sulfonyl group () connected to an amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
group (). Relatively speaking this group is unreactive
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
''Reactivity'' refers to:
* the chemical reactions of a single sub ...
. Because of the rigidity of the functional group, sulfonamides are typically crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
. Many important drugs contain the sulfonamide group.
A sulfonamide (compound) is a chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, R' = R" = hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
) is . Any sulfonamide can be considered as derived from a sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
by replacing a hydroxyl group () with an amine group.
In medicine
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, ...
, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or variation of sulfanilamide. The first sulfonamide was discovered in Germany in 1932.
Synthesis and reactions
Sulfonamides can be prepared in the laboratory in many ways. The classic approach entails the reaction of sulfonyl chlorides with anamine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
.
:
A base such as pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
is typically added to absorb the HCl that is generated. Illustrative is the synthesis of sulfonylmethylamide.
The reaction of primary and secondary amines with benzenesulfonyl chloride is the basis of the Hinsberg reaction, a method for detecting primary and secondary amines.
Sulfonamides undergo a variety of acid-base reactions. The N-H bond can be deprotonated. The alkylsulfonamides can be deprotonated at carbon. Arylsulfonamides undergo ortho-lithiation.
Sultams
Sultams are cyclic sulfonamides. Bioactive sultams include the antiinflammatory ampiroxicam and the anticonvulsant sulthiame. Sultams are prepared analogously to other sulfonamides, allowing for the fact that sulfonic acids are deprotonated by amines. They are often prepared by one-pot oxidation of disulfides or thiols linked to amines. An alternative synthesis of sultams involves initial preparation of a linear sulfonamide, followed by intramolecular C-C bond formation (i.e. cyclization), a strategy that was used in the synthesis of a sultam-based deep-blue emitter fororganic electronics
Organic electronics is a field of materials science concerning the design, Chemical synthesis, synthesis, characterization, and application of Organic compound, organic molecules or polymers that show desirable Electronics, electronic properties ...
.
Sulfanilamide
Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial drug. Chemically, it is an organic compound consisting of an aniline derivatized with a sulfonamide group. Powdered sulfanilamide was used by the Allies in World War ...
, a compound that foreshadowed the development of sulfa drugs
File:Sulfamethoxazole-skeletal.svg, Sulfamethoxazole
Sulfamethoxazole (SMZ or SMX) is an antibiotic. It is used for bacterial infections such as urinary tract infections, bronchitis, and prostatitis and is effective against both gram negative and positive bacteria such as ''Escherichia coli' ...
is a widely used antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
.
File:Ampiroxicam int.svg, Ampiroxicam is a sultam used as an antiinflammatory drug.
File:Hydrochlorothiazide-2D-skeletal.png, Hydrochlorothiazide
Hydrochlorothiazide, sold under the brand name Hydrodiuril among others, is a diuretic medication used to treat hypertension and swelling due to fluid build-up. Other uses include treating diabetes insipidus and renal tubular acidosis and t ...
is a drug that features both acyclic and cyclic sulfonamide groups.
File:Oppolzer sultam.svg , Camphorsultam is a sultam used as a chiral auxiliary in organic synthesis.
Disulfonimides
The disulfonimides are of the type with two sulfonyl groups flanking an amine. As with sulfinamides, this class of compounds is used as catalysts in enantioselective synthesis. Bis(trifluoromethanesulfonyl)aniline is a source of the triflyl () group.See also
* Sulfamide — the sulfonamide parent compound * Sulfamic acid — HOSO2NH2 * Sulfinamide — compounds of the form RS(=O)NR′R″References
Further reading
* Greenwood, David. ''Antimicrobial Drugs: Chronicle of a twentieth century medical triumph'' (Oxford University Press, 2008) popular historysummary
{{Authority control Functional groups