Sulfonated on:
[Wikipedia]
[Google]
[Amazon]
Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid
 Typical conditions involve heating the aromatic compound with sulfuric acid:
:C6H6 + H2SO4 → C6H5SO3H + H2O
Sulfur trioxide or its protonated derivative is the actual
Typical conditions involve heating the aromatic compound with sulfuric acid:
:C6H6 + H2SO4 → C6H5SO3H + H2O
Sulfur trioxide or its protonated derivative is the actual

 Sulfonation of
Sulfonation of
functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
in an electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
. Aryl sulfonic acids are used as detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
s, dye, and drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhal ...
s.
Stoichiometry and mechanism
electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that ca ...
in this electrophilic aromatic substitution.
To drive the equilibrium, dehydrating agents such as thionyl chloride can be added.
:C6H6 + H2SO4 + SOCl2 → C6H5SO3H + SO2 + 2 HCl
Chlorosulfuric acid is also an effective agent:
:C6H6 + HSO3Cl → C6H5SO3H + HCl
In contrast to aromatic nitration and most other electrophilic aromatic substitutions this reaction is reversible. Sulfonation takes place in concentrated acidic conditions and desulfonation is the mode of action in a dilute hot aqueous acid. The reaction is very useful in protecting
Protection is any measure taken to guard a thing against damage caused by outside forces. Protection can be provided to physical objects, including organisms, to systems, and to intangible things like civil and political rights. Although ...
the aromatic system because of this reversibility. Due to their electron withdrawing effects, sulfonate protecting groups can be used to prevent electrophilic aromatic substitution. They can also be installed as directing groups to affect the position where a substitution may take place.T.W> Graham Solomons: ''Organic Chemistry'', 11th Edition, Wiley, Hoboken, NJ, 2013, p. 676, .
Specialized sulfonation methods
Many method have been developed for introducing sulfonate groups aside from direction sulfonation.Piria reaction
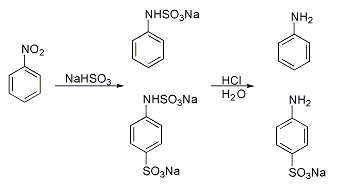
A classic named reaction is the Piria reaction ( Raffaele Piria, 1851) in which nitrobenzene is reacted with a metal bisulfite forming an aminosulfonic acid as a result of combined nitro group reduction and sulfonation.
Tyrer sulfonation process
In the Tyrer sulfonation process (1917), at some time of technological importance, benzene vapor is led through a vessel containing 90% sulfuric acid the temperature of which is increased from 100 to 180°C. Water and benzene are continuously removed in a condenser and the benzene layer fed back to the vessel. In this way an 80% yield is obtained.Applications
Aromatic sulfonic acids are intermediates in the preparation of dyes and many pharmaceuticals. Sulfonation ofaniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile start ...
s lead to a large group of sulfa drug
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimi ...
s.
polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is ...
is used to make sodium polystyrene sulfonate, a common ion exchange resin for water softening.
Reactions of aryl sulfonic acids
As afunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
, aryl sulfonic acids undergo desulfonation when heated in water:
:RC6H4SO3H + H2O → RC6H5 + H2SO4
When treated with strong base, benzenesulfonic acid derivatives convert to phenols.
:C6H5SO3H + 2 NaOH → C6H5OH + Na2SO4 + H2O
See also
*Electrophilic halogenation
In organic chemistry, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution. This organic reaction is typical of aromatic compounds and a very useful method for adding substituents to an aromatic system.
:
A few ...
* Nitration
*Perchlorylbenzene Perchlorylbenzene (C6H5ClO3, PhClO3, is an aromatic compound prepared by direct electrophilic perchlorylation of benzene using perchloryl fluoride and aluminum trichloride:
The compound is described as a somewhat shock-sensitive oily liquid. It ex ...
References
{{DEFAULTSORT:Aromatic Sulfonation Substitution reactions