sodamide on:
[Wikipedia]
[Google]
[Amazon]
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the



inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. It is a salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
composed of the sodium cation and the azanide
Azanide is the IUPAC-sanctioned name for the anion . The term is obscure; derivatives of are almost invariably referred to as amides, despite the fact that amide also refers to the Organic chemistry, organic functional group –. The anion is t ...
anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
. conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. has been widely employed as a strong base
In chemistry, there are three definitions in common use of the word "base": '' Arrhenius bases'', '' Brønsted bases'', and '' Lewis bases''. All definitions agree that bases are substances that react with acids, as originally proposed by G. ...
in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
.
Preparation and structure
Sodium amide can be prepared by the reaction ofsodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
with ammonia gas, but it is usually prepared by the reaction in liquid ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula . A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, ...
using iron(III) nitrate as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. The reaction is fastest at the boiling point of the ammonia, c. −33 °C. An electride
An electride is an ionic compound in which an electron serves the role of the anion. Solutions
Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of a(NH3)6sup>+ and solvated electron ...
, , is formed as a reaction intermediate
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding interme ...
.
:
is a salt-like material and as such, crystallizes as an infinite polymer. The geometry about sodium is tetrahedral. In ammonia, forms conductive solutions, consistent with the presence of and ions.
Uses
Sodium amide is mainly used as astrong base
In chemistry, there are three definitions in common use of the word "base": '' Arrhenius bases'', '' Brønsted bases'', and '' Lewis bases''. All definitions agree that bases are substances that react with acids, as originally proposed by G. ...
in organic chemistry, often suspended (it is insoluble) in liquid ammonia solution. One of the main advantages to the use of sodium amide is its relatively low nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. In the industrial production of indigo
InterGlobe Aviation Limited (d/b/a IndiGo), is an India, Indian airline headquartered in Gurgaon, Haryana, India. It is the largest List of airlines of India, airline in India by passengers carried and fleet size, with a 64.1% domestic market ...
, sodium amide is a component of the highly basic mixture that induces cyclisation of ''N''-phenylglycine. The reaction produces ammonia, which is recycled typically.
Dehydrohalogenation
Sodium amide is a standard base for dehydrohalogenations. It induces the loss of two equivalents ofhydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temper ...
from a vicinal dibromoalkane to give a carbon–carbon triple bond, as in a preparation of phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
In ...
.
Usually two equivalents of sodium amide yields the desired alkyne. Three equivalents are necessary in the preparation of a terminal alkynes because the terminal CH of the resulting alkyne protonates an equivalent amount of base.

Hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
and ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
can also be eliminated in this way, as in the preparation of 1-ethoxy-1-butyne.

Cyclization reactions
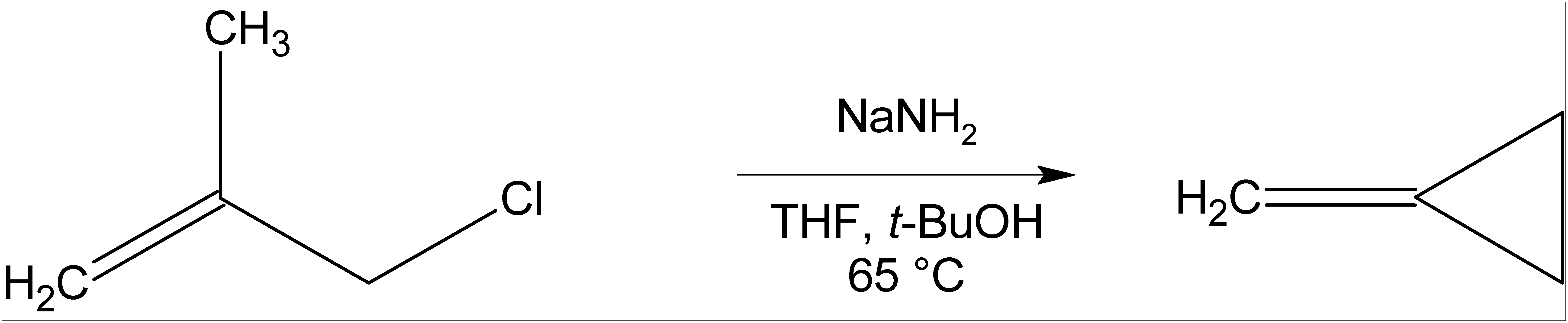
Where there is no β-hydrogen to be eliminated, cyclic compounds may be formed, as in the preparation ofmethylenecyclopropane
Methylenecyclopropane is an organic compound with the formula . It is a hydrocarbon which, as the name suggests, is derived from the addition of a Methylene group, methylene () substituent to a cyclopropane ring. It is a colourless, easily conden ...
below.

Cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental s ...
s, aziridines
and cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commerc ...
s may be formed in a similar manner.
Deprotonation of carbon and nitrogen acids
Carbon acids which can be deprotonated by sodium amide in liquid ammonia include terminalalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s,
methyl ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s,
cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
, phenylacetic acid and its derivatives
and diphenylmethane. Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is classified as a 1,3-diketone. It exists in equilibrium with a tautomer . The mixture is a colorless liquid. These tautomers interconvert so rapidly under most conditions that ...
loses two protons to form a dianion. Sodium amide will also deprotonate indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole ...
and piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor de ...
.
Related non-nucleophilic bases
It is however poorly soluble in solvents other than ammonia. Its use has been superseded by the related reagents sodium hydride, sodium bis(trimethylsilyl)amide (NaHMDS), andlithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
(LDA).
Other reactions
*Rearrangement with orthodeprotonation *Oxirane synthesis *Indole synthesis * Chichibabin reactionSafety
Sodium amide is a common reagent with a long history of laboratory use. It can decompose violently on contact with water, producingammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
:
:
When burned in oxygen, it will give oxides of sodium (which react with the produced water, giving sodium hydroxide) along with nitrogen oxides:
:
:
In the presence of limited quantities of air and moisture, such as in a poorly closed container, explosive mixtures of peroxides may form. This is accompanied by a yellowing or browning of the solid. As such, sodium amide is to be stored in a tightly closed container, under an atmosphere of an inert gas. Sodium amide samples which are yellow or brown in color represent explosion risks.
References
{{Authority control Metal amides Reagents for organic chemistry Sodium compounds