soap film on:
[Wikipedia]
[Google]
[Amazon]
Soap films are thin layers of

 Daily experience shows that soap bubble formation is not feasible with water or with any pure liquid. Actually, the presence of soap, which is composed at a molecular scale of
Daily experience shows that soap bubble formation is not feasible with water or with any pure liquid. Actually, the presence of soap, which is composed at a molecular scale of
 If
If
 During the late stages of draining, sharp-edged black spots start to form. These spots are significantly thinner (< 100 nm) than the normal soap film, giving rise to their black interference colour. Whether black spots can form depends on the concentration of the soap, and moreover there are two types of black films:
* Common black films, around 50 nm in thickness, and
* Newton black films, around 4 nm in thickness, require a higher electrolyte concentration. In these films the outer soap surfaces have effectively snapped together and pinched out most of the inner liquid.
As drainage continues, the black spots eventually take over the entire soap film, and despite its extreme thinness, the final black film can be quite stable and can survive for many minutes.
During the late stages of draining, sharp-edged black spots start to form. These spots are significantly thinner (< 100 nm) than the normal soap film, giving rise to their black interference colour. Whether black spots can form depends on the concentration of the soap, and moreover there are two types of black films:
* Common black films, around 50 nm in thickness, and
* Newton black films, around 4 nm in thickness, require a higher electrolyte concentration. In these films the outer soap surfaces have effectively snapped together and pinched out most of the inner liquid.
As drainage continues, the black spots eventually take over the entire soap film, and despite its extreme thinness, the final black film can be quite stable and can survive for many minutes.
liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
(usually water-based) surrounded by air. For example, if two soap bubble
A soap bubble (commonly referred to as simply a bubble) is an extremely thin soap film, film of soap or detergent and water enclosing air that forms a hollow sphere with an iridescent surface. Soap bubbles usually last for only a few seconds b ...
s come into contact, they merge and a thin film is created in between. Thus, foam
Foams are two-phase materials science, material systems where a gas is dispersed in a second, non-gaseous material, specifically, in which gas cells are enclosed by a distinct liquid or solid material. Note, this source focuses only on liquid ...
s are composed of a network of films connected by Plateau borders. Soap films can be used as model systems for minimal surfaces, which are widely used in mathematics.
Stability

 Daily experience shows that soap bubble formation is not feasible with water or with any pure liquid. Actually, the presence of soap, which is composed at a molecular scale of
Daily experience shows that soap bubble formation is not feasible with water or with any pure liquid. Actually, the presence of soap, which is composed at a molecular scale of surfactants
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a blend of "surface-active agent",
coined in 1950. As t ...
, is necessary to stabilize the film. Most of the time, surfactants are amphiphilic
In chemistry, an amphiphile (), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'', nonpolar) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic c ...
, which means they are molecules with both a hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
and a hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
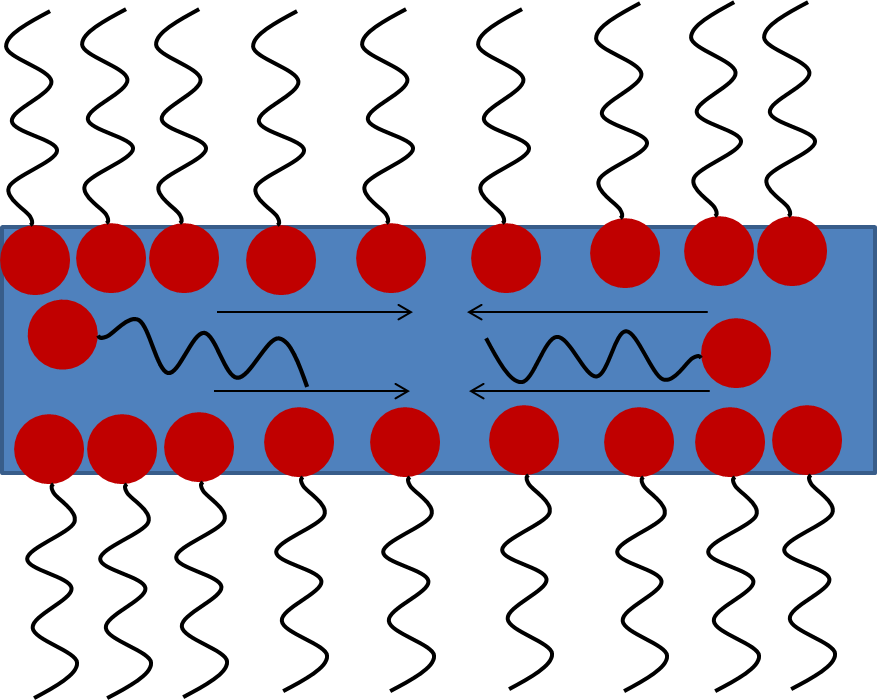
part. Thus, they are arranged preferentially at the air/water interface (see figure 1).
Surfactants stabilize films because they create a repulsion between both surfaces of the film, preventing it from thinning and consequentially bursting. This can be shown quantitatively through calculations relating to disjoining pressure
In surface chemistry, disjoining pressure (symbol ) according to an IUPAC definition arises from an attractive interaction between two surfaces. For two flat and parallel surfaces, the value of the disjoining pressure (i.e., the force per unit are ...
. The main repulsion mechanisms are steric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
(the surfactants can not interlace) and electrostatic (if surfactants are charged).
Moreover, surfactants make the film more stable toward thickness fluctuations due to the Marangoni effect
The Marangoni effect (also called the Gibbs–Marangoni effect) is the mass transfer along an Interface (chemistry), interface between two phases due to a gradient of the surface tension. In the case of temperature dependence, this phenomenon may ...
. This gives some elasticity to the interface: if surface concentrations are not homogeneously dispersed at the surface, Marangoni forces will tend to re-homogenize the surface concentration (see figure 2).
Even in the presence of stabilizing surfactants, a soap film does not last forever. Water evaporates with time depending on the humidity of the atmosphere. Moreover, as soon as a film is not perfectly horizontal, the liquid flows toward the bottom due to gravity and the liquid accumulates at the bottom. At the top, the film thins and bursts.
Importance of surface tension: minimal surfaces
From a mathematical point of view, soap films areminimal surface
In mathematics, a minimal surface is a surface that locally minimizes its area. This is equivalent to having zero mean curvature (see definitions below).
The term "minimal surface" is used because these surfaces originally arose as surfaces that ...
s. Surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Ge ...
is the energy that is required to produce the surface, per unit area. A film—like any body or structure—prefers to exist in a state of minimum potential energy. In order to minimize its energy, a droplet of liquid in free space naturally assumes a spherical shape, which has the minimum surface area for a given volume. Puddle
A puddle is a very small accumulation of liquid, usually water, on a surface. It can form either by pooling in a depression on the surface, or by surface tension upon a flat surface. Puddles are often characterized by murky water or mud due to t ...
s and films can exist in of the presence of other forces, like gravity
In physics, gravity (), also known as gravitation or a gravitational interaction, is a fundamental interaction, a mutual attraction between all massive particles. On Earth, gravity takes a slightly different meaning: the observed force b ...
and the intermolecular attraction to the atoms of a substrate. The latter phenomenon is called wetting
Wetting is the ability of a liquid to displace gas to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. These interactions occur in the presence of either a gaseous phase or ...
: binding forces between the substrate atoms and the film atoms can cause the total energy to decrease. In that case, the lowest energy configuration for the body would be one where as many film atoms as possible are as close as possible to the substrate. That would result in an infinitely thin film, infinitely widely spread out over the substrate. In reality, the effect of adherent wetting (causing surface maximization) and the effect of surface tension (causing surface minimization) would balance each other out: the stable configuration can be a droplet, a puddle, or a thin film, depending on the forces that work on the body.
Colours
Theiridescent
Iridescence (also known as goniochromism) is the phenomenon of certain surfaces that appear gradually to change colour as the angle of view or the angle of illumination changes. Iridescence is caused by wave interference of light in microstruc ...
colours of a soap film are caused by interfering of (internally and externally) reflected light waves, a process called thin film interference and are determined by the thickness of the film. This phenomenon is not the same as the origin of rainbow
A rainbow is an optical phenomenon caused by refraction, internal reflection and dispersion of light in water droplets resulting in a continuous spectrum of light appearing in the sky. The rainbow takes the form of a multicoloured circular ...
colours (caused by the refraction
In physics, refraction is the redirection of a wave as it passes from one transmission medium, medium to another. The redirection can be caused by the wave's change in speed or by a change in the medium. Refraction of light is the most commo ...
of internally reflected light), but rather is the same as the phenomenon causing the colours in an oil slick on a wet road.
Drainage
 If
If surfactant
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a Blend word, blend of "surface-active agent",
coined in ...
s are well chosenBall, 2009. pp. 61–67 and the atmospheric humidity and air movements are suitably controlled, a horizontal soap film can last from minutes to hours. In contrast, a vertical soap film is affected by gravity and so the liquid tends to drain, causing the soap film to thin at the top. Colour depends on film thickness, which accounts for the coloured interference fringes that can be seen at the top of figure 4.
Black spots
 During the late stages of draining, sharp-edged black spots start to form. These spots are significantly thinner (< 100 nm) than the normal soap film, giving rise to their black interference colour. Whether black spots can form depends on the concentration of the soap, and moreover there are two types of black films:
* Common black films, around 50 nm in thickness, and
* Newton black films, around 4 nm in thickness, require a higher electrolyte concentration. In these films the outer soap surfaces have effectively snapped together and pinched out most of the inner liquid.
As drainage continues, the black spots eventually take over the entire soap film, and despite its extreme thinness, the final black film can be quite stable and can survive for many minutes.
During the late stages of draining, sharp-edged black spots start to form. These spots are significantly thinner (< 100 nm) than the normal soap film, giving rise to their black interference colour. Whether black spots can form depends on the concentration of the soap, and moreover there are two types of black films:
* Common black films, around 50 nm in thickness, and
* Newton black films, around 4 nm in thickness, require a higher electrolyte concentration. In these films the outer soap surfaces have effectively snapped together and pinched out most of the inner liquid.
As drainage continues, the black spots eventually take over the entire soap film, and despite its extreme thinness, the final black film can be quite stable and can survive for many minutes.
Bursting
If a soap film is unstable, it ends by bursting. A hole is created somewhere in the film and opens very rapidly. Surface tension indeed leads to surface minimization and, thus, to film disappearance. The hole aperture is not instantaneous and is slowed by the liquid inertia. The balance between the forces of inertia and surface tension leads to the opening velocity: where is the liquid surface tension, is the liquid density and is the film thickness.References
General sources
* {{Foam scales and properties Minimal surfaces Bubbles (physics) he:קרום סבון